Frequently Asked Questions - Institutional Review Board (IRB)
-
What is an IRB?
The primary mission of the Institutional Review Boards (IRB's) is to facilitate ethical research and ensure participant protections by reviewing, approving, modifying or disapproving research applications submitted by UCR researchers. UCR has two local IRBs: IRB-SB (socio-behavioral) and IRB-Clin (clinical-biomedical).
-
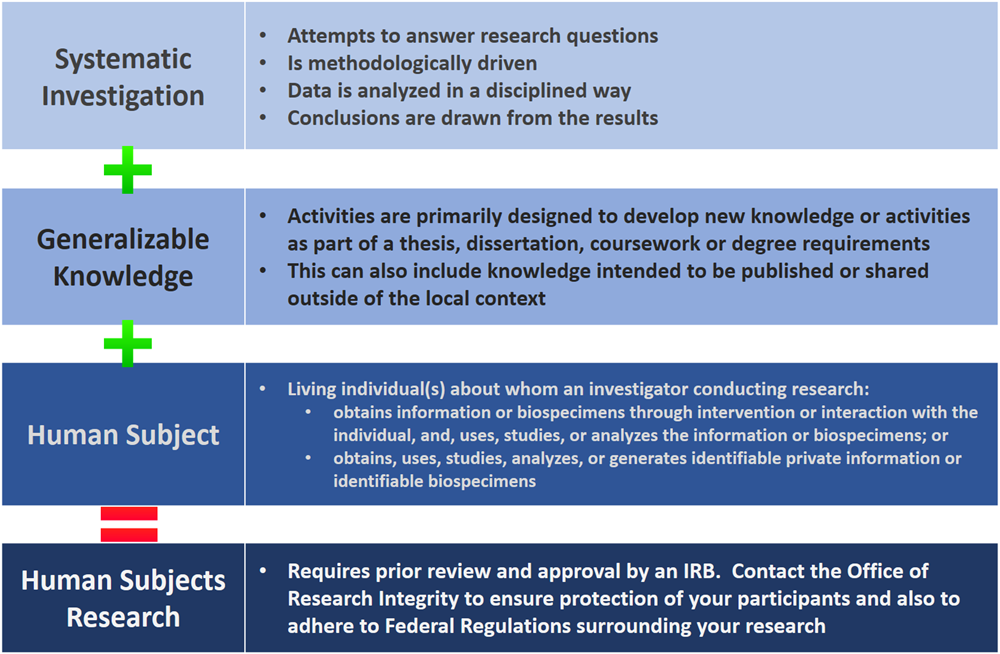
Does my study require IRB review and approval?
The below diagram will help you to determine if the project is ‘human subjects research’ according to federal regulations and therefore requires IRB oversight.
If you are still unsure, we will ask that you fill out a copy of our Determination of Activity Form found on our Forms page and email it to IRB@ucr.edu. This form will give our office a good introduction to your research and whether or not to continue with the full IRB application or if your research does not meet the federal definition of a study requiring IRB oversight.
-
What is meant by the terms "confidential," "de-identified," "coded," and "anonymous"?
The terms confidential, de-identified, coded, and anonymous describe distinct practices and methods related to subjects' participation in research and the collection and storage of participant data.
Participation in research
- An individual’s participation in a research project can be described as anonymous when no one, including the research team, can identify her or him as a participant.
- An individual's participation is considered confidential when the research team knows the individual's identity but is obligated not to disclose that information to others outside of the team (except as clearly described in the research protocol and consent document).
Data collection and storage
- Data are anonymous when no one, including the research team, can connect the data to the individual participant (i.e. no personally identifying information is collected). Keep in mind that even when researchers do not collect direct personal identifiers such as name or student ID number, collection of indirect identifiers, taken in combination, might make it possible for someone to identify an individual participant from among a pool of subjects - for example, academic department and ethnicity.
- Data are confidential when there continues to be a link between the data and the individual who provided it, but the research team is obligated to protect the data from disclosure outside of the research team (except as clearly described in the research protocol and consent document). In order to protect against accidental disclosure, the subject’s name or other identifiers should be stored separately from their research data. In the research data, the identifiers can be replaced with unique codes. Even when data are coded, they are not anonymous because it is still possible to link the codes to identifiable data.
- Data are considered de-identified when any direct or indirect identifiers linking the data to the individual subject’s identity are destroyed.
(source: adapted from http://www.irb.umich.edu/policies/anonymous.pdf)
-
What other documents should I include in my IRB application?
As required as part of the application itself, the following documents should be included as part of the IRB application, as applicable. The ORI may also request additional forms be submitted as part of the application, as well. For example, the following may be requested:
- Project Roster form, list all key personnel as an appendix.
- Informed Consent Form or script (if applicable)
- Study measures including assays or questionnaires
- Researcher Interview Guides or prompts
- Recruitment Materials such as flyers, social media postings, etc.
- Access letters from external sites where research will take place
- Any additional approvals outside of the IRB
-
I have submitted my application, what’s next?
As detailed in the “Categories of Review" sections on the IRB webpage, there are three types of IRB review; Exempt, Expedited and Full Board. The review types are ultimately determined by the Office of Research Integrity and the relevant IRB. During the review process the ORI determines whether the proposed research meets federal criteria for a particular level of review. The levels of IRB review may also change throughout the course of a study, depending on the nature and risk level of subsequent amendments or stages of the research.
Additionally, on the IRB webpage, there is information to help you determine whether your application goes to the IRB-SB or IRB-Clin. For example, the IRB-SB would review a research application for surveys asking about participant health, drug use, or other medical information. Only if a researcher were to conduct this survey and include a blood draw or EKG, for example, would IRB-Clin review be required. -
What are the different types of IRB review?
During its review process, the IRB will assess and evaluate whether the proposed research meets federal criteria for a certain level of review. Remember “Expedited” review doesn’t necessarily mean fast - it simply refers to one of the federal review criteria. There are three levels of federal review: Exempt, Expedited and Full Board. These levels of IRB review may change throughout the course of a study, depending on the nature and risk level of subsequent amendments or the stages of the research.
Exempt review
The exempt review process involves less oversight than an expedited or full-committee review. There are eight federal categories of research activities involving human subjects that may be exempt from the requirements of the Policy for the Protection of Human Subjects (45 CFR 46). At this time, federal exempt categories 7 & 8 are not going to be implemented in whole or in part at any UC campus, including UCR. Projects falling into one of those categories will be reviewed through Expedited procedures.
For additional information, please see the list of Exempt Review Categories.
For Exempt studies, UCR consent or information sheet elements include:- Statement that the study involves research conducted by UCR faculty/student
- Procedures
- Voluntary participation
- Contact information for researchers
- Contact information for UCR IRB
Expedited review
There are 9 federally defined expedited categories. Research activities that (1) present no more than minimal risk to human subjects, and (2) involve only procedures listed in one or more of the Expedited categories, may be reviewed through the expedited review procedure. This review procedure allows the IRB Chairperson or one or more IRB members designated by the Chairperson to evaluate and approve eligible research.
Categories 1 to 7 pertain to both initial and continuing IRB review. Categories 8 and 9 pertain only to continuing IRB review. For additional information, please see the UCR list of Expedited Review Categories.
The most often used Expedited Category at UCR is Expedited #7:
Category 7
Research on individual or group characteristics or behavior (including, but not limited to, research on perception, cognition, motivation, identity, language, communication, cultural beliefs or practices, and social behavior) or research employing survey, interview, oral history, focus group, program evaluation, human factors evaluation, or quality assurance methodologies.
Full Board review
Full Board review is usually reserved for more than minimal risk research. Research that does not fall into either the exempt or expedited review categories must be submitted for full committee review. Please see the Committee Meetings and Submission Deadlines for Full Board Reviews. A study may initially be reviewed at full board level and then determined by the IRB to be eligible for ‘expedited’ review. -
What is the difference then between “Exempt” review and “Not Human Subjects Research” determination?
As part of ORIs evaluation of your research project, we can also determine your application to be “Not Human Subjects Research” or Exempt, Expedited or Full Board. When an application has been determined to be Not Human Subjects Research, it means the IRB has determined that the project is:
- Not about a living individual or
- The investigator is not obtaining information, or biospecimens, about an individual through intervention or interaction, nor obtaining, using, analyzing, or generating, private, identifiable information, or identifiable biospecimens.
- The project is not contributing to generalizable knowledge through a systematic investigation.
When an application has been determined to be Not Human Subjects Research, further oversight of approval by the IRB is not needed. Other types of oversight may be involved.
Oral history projects are likely to be determined to be “Not Human Subjects Research” depending on their scope. Simulations of human experimentation and course-assigned data collection do not constitute human subjects research if the activities are designed for educational purposes only and will not be further published, presented or contribute to generalizable knowledge.
There may be instances in which a student or instructor wishes to perform research on data that was previously collected for educational purposes. An application should be submitted to the IRB whenever a student or instructor wishes to analyze the data with the intent of contributing to generalizable knowledge. -
What does it mean to be 'Engaged in Research'?
According to the Office for Human Research Protections (OHRP), an institution/entity is considered to be engaged in human subjects research (HSR) when its personnel or agents for the purposes of the research:
- Receive an award through a grant, contract, or cooperative agreement directly from HHS;
- Intervene for research purposes with any human subjects of the research by performing invasive or noninvasive procedures;
- Intervene by manipulating the environment;
- Interact with subjects for research purposes;
- Obtain consent of subjects;
- Obtain, for research purposes, identifiable private information or identifiable biological specimens from any source
Example #1: UCR Researcher is interested in conducting a national survey about voter turnout in minority communities. As part of this project, the researcher will conduct interviews with individuals in different states. The researcher sub-contracts with a survey firm to collect the survey data. The firm will obtain informed consent. Both UCR and the survey firm are enaged in HSR and require IRB approval.
Example #2: A UCR Researcher initiates a collaborative research project that includes a blood draw and medical record reviews with a health clinic in San Bernardino. The Health Clinic will provide the medical record information as well as perform the blood draw once informed consent has been received. Both UCR and the Health Clinic are engaged in HSR and require IRB approval.
For additional information, please see the HHS guidance on Engagement of Institutions in Human Subjects Research. -
Members of my research team need to take the required human subjects training. Where can they find that?
This information can be found on both the IRB webpage and here in this FAQ. All investigators and staff conducting human subjects' research are required to complete the Collaborative Institutional Training Initiative (CITI) course. Review the CITI training instructions.
-
Do my Faculty Advisor (if student) & Department Chair really need to sign the IRB application?
Yes. Additionally ORI does accept electronic signatures on applications if they are scanned and sent to us via email. As most applications are sent via email we ask that you do not drop a copy off to our office location. Signature requirements also apply to amendments & continuing approvals as listed on the application itself.
-
If my study application needs to go to a Full Board review, what are the meeting dates?
You can find the meeting calendars for UCR IRB on the IRB webpage webpage. Please note these submission dates apply for studies deemed to be more than minimal risk. All other IRB requests are accepted on rolling submissions.
-
Why does it make a difference if my study receives federal funding?
If you receive federal funding for your research involving human subjects, we must follow the federal Common Rule regulations (45 CFR 46) when reviewing your study. In addition to those regulations, certain federal agencies have mandated additional requirements included as part of a research approval by the IRB. These agencies include the DoD, DoE, DoJ, ED and EPA. Study review and approval may take additional time to account for the requirements of each of these agencies.
-
Can I offer a raffle or drawing as an incentive to participate in my research?
Yes, but your raffle or drawing must be designed so that it complies with State Law.
UCOP interprets California State Law prohibiting lotteries as also prohibiting compensation to research participants through a drawing. A lottery occurs when the individual included in the drawing provides something of value in return for their inclusion. In cases where research participants are entered into a drawing, the participants’ participation is something of value. So, if inclusion in the drawing is dependent upon participation, California law may determine the drawing meets the definition of “lottery”, an activity that is prohibited.
One method of ensuring the drawing is not a lottery is to open the drawing to everyone, regardless of their participation. You can revise the application, study advertisements and consent form to include a mechanism for those who do not participate in the study to enter the drawing. For example, you could add the following language to the invitation:“Everyone can be entered in the drawing regardless of participation. If you do not want to participate but want to be included in the drawing, please email me at XXXX@ucr.edu.”
Data show that not many people will pursue inclusion in the drawing when they do not participate. -
Are you available to meet with me to discuss my research?
Yes, we do offer compliance consults to our researchers. Please email us at IRB@ucr.edu to set up an appointment to speak with an ORI representative. Please provide as much information about your request as possible so we can arrange the meeting with the right people at ORI.
-
My study is ongoing and I need to update or add a procedure, survey, interview questions, subjects, personnel, a research site, compensation, etc. Do I need to inform the IRB?
Yes. Any time you would like to add something new to the study, whether it is a procedural change or a new question or set of questions, an amendment is required. Please fill out an Amendment Request Form found in the IRB section of the Forms Page and submit it to the IRB for review. Once it is reviewed and approved, an approval email will be sent to you, and only then can the changes be implemented.
This requirement pertains to all applications, whether approved as Exempt, Expedited or Full Board. However, please note that changes that only impact the Project Roster (personnel changes), do not require the submission of the Amendment request form. Email the irb@ucr.edu directly with these changes.
-
When should I close out my IRB application?
It is the responsibility of the Principal Investigator to comply with continuing review requirements and submit required documentation in a timely manner in order to ensure that there will be no interruption in the research process. The Principal Investigator must ensure that their protocol does not meet or exceed the expiration date that has been initially approved.
For applicable studies, the Office of Research Integrity sends an expiration notification to the principal investigator 60 days prior to study expiration. However, the Principal Investigator will have ultimate responsibility for ansuring a timely submission of their protocol information. The required documentation for renewal must be completed and submitted to the ORI 30 days in advance of the next IRB meeting date to account for a timely pre-review of submitted materials. -
What is HIPAA and what do I need to do about it?
Anyone conducting research dealing with HIPAA also needs to complete the HIPAA tutorial. You will need to register in order to complete the tutorial.
When research involves the use or disclosure of PHI by entities subject to the regulations, the rules will apply. Researchers have legitimate needs to use, access, and disclose PHI to carry out a wide range of health research studies. In most instances, the Privacy Rule requires an authorization from the individual or a waiver of authorization from an IRB or Privacy Board before a covered entity can access, use or disclose PHI for research purposes. In general, there are two types of human research that would involve PHI:- Studies involving review of medical records as a source of research information.
- Studies that create new medical information because a health care service is being performed as part of the research.
The UCR IRB will approve either:
- A full waiver of authorization to conduct all the research activities described in the research proposal; or
- A partial waiver of authorization for specific research actives such as recruitment.
In most instances, a full waiver of authorization is granted only when there is no opportunity for the researcher to obtain authorization from the individual. Partial waivers of authorization are often granted to allow researchers to access the EMR to identify potential research participants.
-
Can I conduct human subjects research using prisoners as participants?
Yes; however, in order to conduct research with prisoners, researchers must adhere to additional regulations beyond the basic requirements for research with human subjects. Research with prisoners is governed by federal regulations that classify prisoners as a “vulnerable population” (45 CFR 46 Subpart C) because their ability to make an informed and voluntary decision to participate in research is compromised by their incarceration.
Certain types of research with prisoners are permissible but often require review and approval from several agencies including the prison facility. In the state of California, research is governed by the Research Review Process for the California Department of Corrections and Rehabilitation (CDCR). Additionally, the state of California has a penal code (3502a) prohibition on any biomedical research conducted on prisoners in the state. For this reason, the UCR IRB will not approve prisoners to be involved in any biomedical research studies. Biomedical research is defined by CA law as, “research relating to or involving biological, medical or physical science.” Authorization from the DHHS OHRP must be obtained prior to the initiation of any federally funded research involving prisoners.
Researchers are strongly encouraged to contact the IRB during the design of any research studies that may involve prisoners.
Definition of Prisoner
A prisoner, as defined by Federal Regulation (45 CFR 46.303 (c)) is any individual involuntarily confined or detained in a penal institution, including:- individuals sentenced to such an institution under a criminal or civil statute;
- individuals detained in other facilities by virtue of statutes or commitment procedures which provide alternatives to criminal prosecution or incarceration in a penal institution and;
- individuals detained pending arraignment, trial, or sentencing
Common examples of the application of the regulatory definition of prisoner are as follows:
- Individuals who are detained in a residential facility for court-ordered substance abuse treatment as a form of sentencing or alternative to incarceration are prisoners
- Individuals with psychiatric illnesses who have been committed involuntarily to an institution as an alternative to a criminal prosecution or incarceration are prisoners
- Parolees who are detained in a treatment center as a condition of parole are prisoners
Common examples of individuals who are not defined as prisoners according to the regulations:
- Individuals who are receiving non-residential court-ordered substance abuse treatment and are residing in the community;
- Individuals who have been voluntarily admitted to an institution for treatment of a psychiatric illness, or who have been civilly committed to non-penal institution because of their illness makes them a danger to themselves or others;
- Individuals living in the community and sentenced to community-supervised monitoring, including parolees, are not prisoners;
- Probationers and individuals wearing monitoring devices are generally not considered to be prisoners; however, situations of this kind frequently require an analysis of the particular circumstances of the planned subject population. Institutions may consult with OHRP when questions arise about research involving these populations.
Types of Permissible Research with Prisoners
On a case by case basis, the IRB must decide which one of the categories listed below best represents the proposed research:- §46.306(a)(2)(i): A study of the possible causes, effects, and processes of incarceration, and of criminal behavior, provided that the study presents no more than minimal risk and no more than inconvenience to the participants;
- §46.306(a)(2)(ii): A study of prisons as institutional structures or of prisoners as incarcerated persons, provided that the study presents no more than minimal risk and no more than inconvenience to participants;
- §46.306(a)(2)(iii): Research on conditions particularly affecting prisoners as a class (e.g., vaccine trials and other research on hepatitis which is much more prevalent in prisons than elsewhere; and research on social and psychological problems such as alcoholism, drug addictions, and sexual assaults);
- §46.306(a)(2)(iv): Research on practices, both innovative and accepted, which have the intent and reasonable probability of improving the health or well-being of the participant.
Federally funded research falling in categories iii and iv may only proceed after the HHS Secretary has consulted with appropriate experts, including experts in penology, medicine, and ethics, and has published notice in the Federal Register of his or her intent to approve the research.
When a Participant Becomes Incarcerated during a Study
If an ongoing study requires a visit with a participant who has become incarcerated during the course of the research, the researcher must cease all contact with the participant during his/her incarceration. Any data collected prior to the incarceration may still be used; however, new data may not be collected. If a study necessitates visiting the participant while they are incarcerated, the researcher must amend their IRB application to include prisoners as part of their participant population. The amendment will then be reviewed by the IRB according to the additional required regulations. -
I am a non-UC researcher at another campus who would like to access UCR participants, what do I need to do?
UCR does have a process that allows non-UC PIs to request approval to use UCR’s students, faculty and/or staff. The first step is to complete the UCR Administrative Review for Human Research Studies Being Conducted by Non-UCR Principal Investigators Accessing UCR Facilities, Patients or Personnel and submit that along with your approved IRB application (and its relevant materials [consent/survey/flyer/ad, etc.]), mention a UCR faculty member who’s assisting you (if applicable), and verification of human subjects training program to IRB@ucr.edu.
-
I want to start a drug trial, device trial or am not sure if my research with a drug or newly developed device falls under the purview of FDA regulated or clinical trials. Can you help me?
Yes. Depending on the type of research you are conducting and the type of product (if any) that has been developed, your research with any new App, drug, device, software, etc. may be subject to additional regulations with additional regulatory agencies (e.g. FDA, DoD). Please contact the ORI at IRB@ucr.edu to begin the application process to determine which federal regulations may apply to your research.
-
What is a “waiver” of informed consent and how do I apply for one?
A waiver of informed consent could: 1) alter some or all of the required elements of informed consent or 2) completely waive the requirement to obtain informed consent. The IRB may approve a consent procedure which does not include or alters some or all of the required elements of informed consent provided all of the following have been determined to be true by the IRB:
- The research involves no more than minimal risk
- The waiver of informed consent will not adversely affect the rights and welfare of the subjects
- It is not practicable to conduct the research without the waiver or alteration
- If the research involves using identifiable private information or identifiable biospecimens, the research could not be practicably carried out without using such information or biospecimens in an identifiable format.
- Whenever appropriate, participants will be provided with additional pertinent information after their participation.
Examples of types of studies in which some or all elements of consent have been waived include retrospective chart reviews, studies of existing specimens, ethnographic research, studies that require deception or passive (opt-out) consent. Also keep in mind that waiver of informed consent is where some or all elements of consent is removed. This is fundamentally different than a waiver of documentation of informed consent (see FAQ #19) where only the signature of the participant on the inform consent document is waived. I.e. only survey forms where a signed document would be difficult to obtain.
-
What is a waiver of documentation of informed consent?
A waiver of documentation of informed consent alters the informed consent process by eliminating the requirement for research participants to sign the informed consent form. This means that researchers are still required to provide participants with the required informed consent information, but a signature is not obtained. Investigators requesting this waiver will be required to include in their IRB application the consent information that will be provided to the participants and details on how consent will be documented in lieu of the signed form (e.g., investigators will include in their research notes that participant ‘X’ provided consent).
The IRB may approve a consent procedure which waives the documentation of informed consent provided the IRB finds the research to meet the following:- The only record linking the participant and the research would be the consent document;
- The principal risk would be potential harm resulting from a breach of confidentiality; and
- Additionally, each participant will be asked whether the participant wants documentation linking the participant with the research, and the participant’s wishes will govern;
OR - The research is minimal risk and
- Involves no procedures that usually require written consent;
OR - The participants or legally authorized representatives are members of a distinct cultural group or community in which signing forms is not the norm;
- The research presents no more than minimal risk of harm to participants; and
- There is an appropriate alternative mechanism for documenting that informed consent was obtained.
Examples of studies where the documentation requirement have been waived include studies on sensitive topics such as violence or illegal activities and studies where participants may not be interacting directly with the investigators such as telephone or web-based surveys.
-
What are Short Forms?
A short is a written document stating that the elements of informed consent required by 45 CFR 46.117 have been presented to and understood by the subject or the subject's legally authorized representative.
A short form may be used when the majority of subjects in a study are English speakers, but there are a portion of the subjects who will not be able to understand the consent form written in English. A short form can be used in these instances to obtain consent ensuring equal access for potential participants.
If the majority of the anticipated subjects to be enrolled do not speak English or will be unable to understand the consent form written in English, the consent form must be translated into a language understandable to the subjects. When the person obtaining consent is assisted by a translator, the translator may serve as a witness. The subject must be given copies of the short form document and the summary.
A short form may be used in conjunction with an oral presentation of the consent information required by 45 CFR 46.117. A summary of what will be said to the subject or representative must be approved by the IRB and then presented orally to the subject or representative in front of a witness. A copy of the short form and a copy of the summary must be given to the subject or representative.
Required signatures with short forms- Short Form - Subject/Representative and the Witness
- Copy of Summary - Witness and Person obtaining consent
In a research application, the UCR IRB must receive justification for the use of short forms, a summary of what will be presented to the subject or representative and the text of your short form.
-
I’m conducting a project which uses an Oral History methodology, do I need IRB review?
It depends. Determining whether a project using oral history methodologies requires IRB review involves evaluating whether the investigator is engaged in the creation of "generalizable knowledge".
Federal regulations define “research” as “a systematic investigation, including research development, testing, and evaluation, designed to develop or contribute to generalizable knowledge.” The 2018 revision of the Common Rule (45 CFR 46) included a provision to clarify that certain activities are excluded from the federal “research” definition. These activities include:
Scholarly and journalistic activities (e.g., oral history, journalism, biography, literary criticism, legal research, and historical scholarship), including the collection and use of information, that focus directly on the specific individuals about whom the information is collected (45 CFR 46.102(l)(1)).
Oral history activities intended to create a record of specific historical events and/or provide an accurate and evidence-based portrayal of the individuals involved are not meant to contribute to generalizable knowledge and hence do not require IRB review.
If, however, the project uses oral history activities designed with the intent to collect information and draw general conclusions, inform policy or test a hypothesis, then this project would require IRB review. Researchers must complete and submit the General IRB Application Form and Project Roster to irb@ucr.edu for review.
Examples:- An oral history video recording of interviews with World War II survivors is created for viewing in the WWII Museum. The purpose is to create a historical record of specific personal events and experiences related to the War and provide a venue for survivors to tell their stories. The creation of the videotape is not intended to prove a hypothesis, inform policy, or draw conclusions. Therefore, this project would not constitute research requiring IRB review.
- Oral history interviews are conducted with surviving Gulf War veterans to document their experiences in order to draw conclusions about those experiences, inform policy, and generalize findings. This project would constitute research requiring IRB review.
Remember, you can always contact the IRB for a consultation to discuss your project by emailing irb@ucr.edu.
-
When do I need to give the Experimental Subject's Bill of Rights form to my research participants?
California law, under Health & Safety Code §24172, requires all investigators doing a "medical experiment" to offer their subjects a copy of the "Experimental Subject's Bill of Rights." Failure to do so may result in civil or criminal penalties. To ensure compliance with California law, investigators should document that each subject received the form using one of the methods described below.
A "medical experiment" is defined as:
The severance or penetration or damaging of tissues of a human subject, or the use of a drug or device as defined in section 109920 of 109925 (of the Health and Safety Code), electromagnetic radiation, heat or cold, or a biological substance or organism, in or upon a human subject in the practice or research of medicine in a manner not reasonably related to maintaining or improving the health of such subject or otherwise directly benefiting such subject. The investigational use of a drug or device as provided in Sections 111590 and 111595. Withholding medical treatment from a human subject for any purpose other than maintenance or improvement of the health of the subject.
UC has interpreted this definition to include almost all studies involving biomedical procedures, placebo controls, innovative therapy and/or normal volunteer subjects.
For these types of studies, you must give a copy of the UCR Experimental Subject's Bill of Rights to subjects in a language in which the subject is fluent, along with a copy of the study's consent document and possibly the HIPAA Authorization Form should the study include collection of protected health information (PHI).
For non-biomedical studies, the IRB may recommend use of the Experimental Subject's Bill of Rights, though it is not required by law.
How Should Researchers Document Dispensation of the Form?
Subjects do not sign the Experimental Subject's Bill of Rights, but you should document that each subject received the form by one of the following methods:- Keep a copy of the Bill of Rights with the subject’s initials in his/her study file;
- Write a note on the consent form confirming that the subject received the Bill of Rights;
- Write a note in the subject’s research record confirming that the subject received the Bill of Rights; OR
- Keep a copy of the Bill of Rights in the subject’s research file with the original signed consent form (and HIPAA authorization form, if applicable).
Please do NOT include the Bill of Rights in the appendices of your IRB application.