Research Compliance

Responsibilities of ORC
We recently transitioned to Kuali's online applications to help streamline the management of research protocols. For details and training resources, visit UCR's Kuali Research Page.
The Office of Research Compliance (ORC) provides broad oversight, resources and education for integrity and compliance issues relating to the conduct of research at the University of California, Riverside. We strive to promote excellence in research while ensuring compliance with federal and state regulations. The Office of Research Compliance has oversight and responsibility over the IRB, several committees, as well as educating and training researchers on research integrity and compliance and responding to Allegations of Research Misconduct (RM).
Committees
The Institutional Biosafety Committee (IBC) was formerly administered by ORC personnel. Those duties have been transferred to Environmental Health and Safety (EH&S).
Institutional Review Board (IRB)
The Institutional Review Board (IRB) functions as the review body for the approval and oversight of all human subjects research conducted on behalf of the institution. The function of the IRB is to ensure adherence to all federal, state, local, and institutional regulations concerning the protection of human subjects. UCR IRB review is required for both funded and non-funded human subjects research.
Institutional Animal Care and Use Committee (IACUC)
Administering and supporting functions of Institutional Animal Care and Use Committee (IACUC), which has authority over all activities involving vertebrate animals and care for research and teaching
Conflict of Interest (COI)
The Conflict of Interest (COI) committee is charged with reviewing investigator statements of financial interest related to their sponsored research activities and determining whether a conflict of interest management plan is warranted after review of all the facts and circumstances.
Stem Cell Research Oversight (SCRO)
The Stem Cell Research Oversight (SCRO) committee reviews activities involving human stem cell research, regardless of the type of stem cells or whether the stem cells are adult or embryonic.
Dual Use Research of Concern (DURC)
The Dual Use Research of Concern (DURC) policy is intended to strengthen the University’s institutional review and oversight of life sciences research to identify potential DURC, and to develop and implement risk mitigation where appropriate and as required by federal regulation.
Export Control
The Export Control Office assists UCR faculty, academic appointees, staff, students, including student employees and non-employee participants in UCR programs in their understanding of export control matters to ensure continued compliance with applicable export control requirements.
Research Compliance Series
Our 2022-2023 series discussed recent NIH and upcoming NSF changes to requirements for education in the responsible conduct of research [RCR], and an examination of what our campus wide responsibilities are to be.
-
View session recordings
October 20, 2022 ››
Password: 9V2t9pm@November 17, 2022 ››
Password: n&EG3%qm (You must complete the registration form to access the recording.)January 19, 2023 ››
Password: u+?874Vc (You must complete the registration form to access the recording.)February 16, 2023 ››
Passcode: Te0qs7G$ (You must complete the registration form to access the recording.)
-
Institutional Registration and Accreditation Numbers
Institutional Human Subjects Assurance
Institutional Federal-Wide Assurance #00001965
Effective Dates: May 2, 2023 through May 2, 2028
IORG-IRB Registration
IRB (Human Subjects) registration #IRB00000195
Effective Dates: May 2, 2023 through May 2, 2026
Institutional Lab Animal Assurance
Institutional number: D16-00278 (A3439-01)
Effective Dates: August 15, 2023 through August 31, 2027
Renewed every four years
AAALAC Accreditation
Institutional number: 000637
AAALAC Accreditation: 1986
Renewed every three years - most recent renewal: July 7, 2021
Research Misconduct Assurance
Institutional number: 0577506
Effective January 3, 2012
Renewed annually
US Department of Agriculture
Registration number: 93-R-0436
USDA Type of Performing Institution Designation
Class R – Research Facility

Contacts
| ORC Function | Contact |
|---|---|
| IRB | irb@ucr.edu |
| IACUC - inquiries | iacuc@ucr.edu |
| IACUC - Animal User Training | iacuctraining@ucr.edu |
| COI | coic@ucr.edu |
| SCRO | scro@ucr.edu |
| DURC | Sherie Donahue |
| Kuali Training | kualitraining@ucr.edu |
Institutional Review Board (IRB) FAQs
-
What is an IRB?
The primary mission of the Institutional Review Boards (IRB's) is to facilitate ethical research and ensure participant protections by reviewing, approving, modifying or disapproving research applications submitted by UCR researchers. UCR has two local IRBs: IRB-SB (socio-behavioral) and IRB-Clin (clinical-biomedical).
-
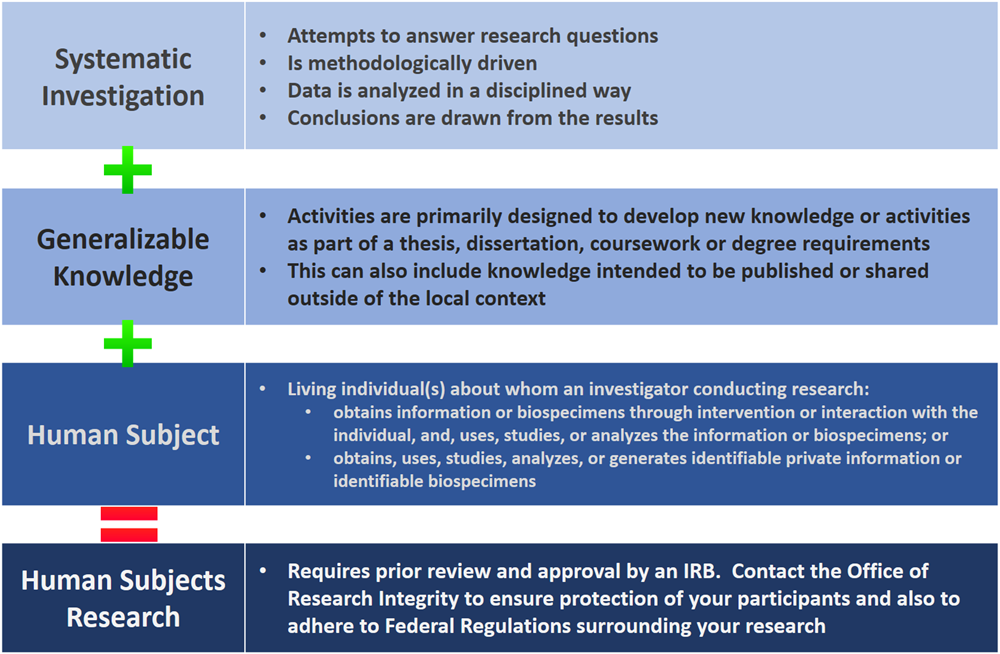
Does my study require IRB review and approval?
The below diagram will help you to determine if the project is ‘human subjects research’ according to federal regulations and therefore requires IRB oversight.
If you are still unsure, we will ask that you fill out a copy of our Determination of Activity Form found on our Forms page and email it to IRB@ucr.edu. This form will give our office a good introduction to your research and whether or not to continue with the full IRB application or if your research does not meet the federal definition of a study requiring IRB oversight.
-
What is meant by the terms "confidential," "de-identified," "coded," and "anonymous"?
The terms confidential, de-identified, coded, and anonymous describe distinct practices and methods related to subjects' participation in research and the collection and storage of participant data.
Participation in research
- An individual’s participation in a research project can be described as anonymous when no one, including the research team, can identify her or him as a participant.
- An individual's participation is considered confidential when the research team knows the individual's identity but is obligated not to disclose that information to others outside of the team (except as clearly described in the research protocol and consent document).
Data collection and storage
- Data are anonymous when no one, including the research team, can connect the data to the individual participant (i.e. no personally identifying information is collected). Keep in mind that even when researchers do not collect direct personal identifiers such as name or student ID number, collection of indirect identifiers, taken in combination, might make it possible for someone to identify an individual participant from among a pool of subjects - for example, academic department and ethnicity.
- Data are confidential when there continues to be a link between the data and the individual who provided it, but the research team is obligated to protect the data from disclosure outside of the research team (except as clearly described in the research protocol and consent document). In order to protect against accidental disclosure, the subject’s name or other identifiers should be stored separately from their research data. In the research data, the identifiers can be replaced with unique codes. Even when data are coded, they are not anonymous because it is still possible to link the codes to identifiable data.
- Data are considered de-identified when any direct or indirect identifiers linking the data to the individual subject’s identity are destroyed.
(source: adapted from http://www.irb.umich.edu/policies/anonymous.pdf)
-
What other documents should I include in my IRB application?
As required as part of the application itself, the following documents should be included as part of the IRB application, as applicable. The ORI may also request additional forms be submitted as part of the application, as well. For example, the following may be requested:
- Project Roster form, list all key personnel as an appendix.
- Informed Consent Form or script (if applicable)
- Study measures including assays or questionnaires
- Researcher Interview Guides or prompts
- Recruitment Materials such as flyers, social media postings, etc.
- Access letters from external sites where research will take place
- Any additional approvals outside of the IRB
-
I have submitted my application, what’s next?
As detailed in the “Categories of Review" sections on the IRB webpage, there are three types of IRB review; Exempt, Expedited and Full Board. The review types are ultimately determined by the Office of Research Integrity and the relevant IRB. During the review process the ORI determines whether the proposed research meets federal criteria for a particular level of review. The levels of IRB review may also change throughout the course of a study, depending on the nature and risk level of subsequent amendments or stages of the research.
Additionally, on the IRB webpage, there is information to help you determine whether your application goes to the IRB-SB or IRB-Clin. For example, the IRB-SB would review a research application for surveys asking about participant health, drug use, or other medical information. Only if a researcher were to conduct this survey and include a blood draw or EKG, for example, would IRB-Clin review be required. -
What are the different types of IRB review?
During its review process, the IRB will assess and evaluate whether the proposed research meets federal criteria for a certain level of review. Remember “Expedited” review doesn’t necessarily mean fast - it simply refers to one of the federal review criteria. There are three levels of federal review: Exempt, Expedited and Full Board. These levels of IRB review may change throughout the course of a study, depending on the nature and risk level of subsequent amendments or the stages of the research.
Exempt review
The exempt review process involves less oversight than an expedited or full-committee review. There are eight federal categories of research activities involving human subjects that may be exempt from the requirements of the Policy for the Protection of Human Subjects (45 CFR 46). At this time, federal exempt categories 7 & 8 are not going to be implemented in whole or in part at any UC campus, including UCR. Projects falling into one of those categories will be reviewed through Expedited procedures.
For additional information, please see the list of Exempt Review Categories.
For Exempt studies, UCR consent or information sheet elements include:- Statement that the study involves research conducted by UCR faculty/student
- Procedures
- Voluntary participation
- Contact information for researchers
- Contact information for UCR IRB
Expedited review
There are 9 federally defined expedited categories. Research activities that (1) present no more than minimal risk to human subjects, and (2) involve only procedures listed in one or more of the Expedited categories, may be reviewed through the expedited review procedure. This review procedure allows the IRB Chairperson or one or more IRB members designated by the Chairperson to evaluate and approve eligible research.
Categories 1 to 7 pertain to both initial and continuing IRB review. Categories 8 and 9 pertain only to continuing IRB review. For additional information, please see the UCR list of Expedited Review Categories.
The most often used Expedited Category at UCR is Expedited #7:
Category 7
Research on individual or group characteristics or behavior (including, but not limited to, research on perception, cognition, motivation, identity, language, communication, cultural beliefs or practices, and social behavior) or research employing survey, interview, oral history, focus group, program evaluation, human factors evaluation, or quality assurance methodologies.
Full Board review
Full Board review is usually reserved for more than minimal risk research. Research that does not fall into either the exempt or expedited review categories must be submitted for full committee review. Please see the Committee Meetings and Submission Deadlines for Full Board Reviews. A study may initially be reviewed at full board level and then determined by the IRB to be eligible for ‘expedited’ review. -
What is the difference then between “Exempt” review and “Not Human Subjects Research” determination?
As part of ORIs evaluation of your research project, we can also determine your application to be “Not Human Subjects Research” or Exempt, Expedited or Full Board. When an application has been determined to be Not Human Subjects Research, it means the IRB has determined that the project is:
- Not about a living individual or
- The investigator is not obtaining information, or biospecimens, about an individual through intervention or interaction, nor obtaining, using, analyzing, or generating, private, identifiable information, or identifiable biospecimens.
- The project is not contributing to generalizable knowledge through a systematic investigation.
When an application has been determined to be Not Human Subjects Research, further oversight of approval by the IRB is not needed. Other types of oversight may be involved.
Oral history projects are likely to be determined to be “Not Human Subjects Research” depending on their scope. Simulations of human experimentation and course-assigned data collection do not constitute human subjects research if the activities are designed for educational purposes only and will not be further published, presented or contribute to generalizable knowledge.
There may be instances in which a student or instructor wishes to perform research on data that was previously collected for educational purposes. An application should be submitted to the IRB whenever a student or instructor wishes to analyze the data with the intent of contributing to generalizable knowledge. -
What does it mean to be 'Engaged in Research'?
According to the Office for Human Research Protections (OHRP), an institution/entity is considered to be engaged in human subjects research (HSR) when its personnel or agents for the purposes of the research:
- Receive an award through a grant, contract, or cooperative agreement directly from HHS;
- Intervene for research purposes with any human subjects of the research by performing invasive or noninvasive procedures;
- Intervene by manipulating the environment;
- Interact with subjects for research purposes;
- Obtain consent of subjects;
- Obtain, for research purposes, identifiable private information or identifiable biological specimens from any source
Example #1: UCR Researcher is interested in conducting a national survey about voter turnout in minority communities. As part of this project, the researcher will conduct interviews with individuals in different states. The researcher sub-contracts with a survey firm to collect the survey data. The firm will obtain informed consent. Both UCR and the survey firm are enaged in HSR and require IRB approval.
Example #2: A UCR Researcher initiates a collaborative research project that includes a blood draw and medical record reviews with a health clinic in San Bernardino. The Health Clinic will provide the medical record information as well as perform the blood draw once informed consent has been received. Both UCR and the Health Clinic are engaged in HSR and require IRB approval.
For additional information, please see the HHS guidance on Engagement of Institutions in Human Subjects Research. -
Members of my research team need to take the required human subjects training. Where can they find that?
This information can be found on both the IRB webpage and here in this FAQ. All investigators and staff conducting human subjects' research are required to complete the Collaborative Institutional Training Initiative (CITI) course. Review the CITI training instructions.
-
Do my Faculty Advisor (if student) & Department Chair really need to sign the IRB application?
Yes. Additionally ORI does accept electronic signatures on applications if they are scanned and sent to us via email. As most applications are sent via email we ask that you do not drop a copy off to our office location. Signature requirements also apply to amendments & continuing approvals as listed on the application itself.
-
If my study application needs to go to a Full Board review, what are the meeting dates?
You can find the meeting calendars for UCR IRB on the IRB webpage webpage. Please note these submission dates apply for studies deemed to be more than minimal risk. All other IRB requests are accepted on rolling submissions.
-
Why does it make a difference if my study receives federal funding?
If you receive federal funding for your research involving human subjects, we must follow the federal Common Rule regulations (45 CFR 46) when reviewing your study. In addition to those regulations, certain federal agencies have mandated additional requirements included as part of a research approval by the IRB. These agencies include the DoD, DoE, DoJ, ED and EPA. Study review and approval may take additional time to account for the requirements of each of these agencies.
-
Can I offer a raffle or drawing as an incentive to participate in my research?
Yes, but your raffle or drawing must be designed so that it complies with State Law.
UCOP interprets California State Law prohibiting lotteries as also prohibiting compensation to research participants through a drawing. A lottery occurs when the individual included in the drawing provides something of value in return for their inclusion. In cases where research participants are entered into a drawing, the participants’ participation is something of value. So, if inclusion in the drawing is dependent upon participation, California law may determine the drawing meets the definition of “lottery”, an activity that is prohibited.
One method of ensuring the drawing is not a lottery is to open the drawing to everyone, regardless of their participation. You can revise the application, study advertisements and consent form to include a mechanism for those who do not participate in the study to enter the drawing. For example, you could add the following language to the invitation:“Everyone can be entered in the drawing regardless of participation. If you do not want to participate but want to be included in the drawing, please email me at XXXX@ucr.edu.”
Data show that not many people will pursue inclusion in the drawing when they do not participate. -
Are you available to meet with me to discuss my research?
Yes, we do offer compliance consults to our researchers. Please email us at IRB@ucr.edu to set up an appointment to speak with an ORI representative. Please provide as much information about your request as possible so we can arrange the meeting with the right people at ORI.
-
My study is ongoing and I need to update or add a procedure, survey, interview questions, subjects, personnel, a research site, compensation, etc. Do I need to inform the IRB?
Yes. Any time you would like to add something new to the study, whether it is a procedural change or a new question or set of questions, an amendment is required. Please fill out an Amendment Request Form found in the IRB section of the Forms Page and submit it to the IRB for review. Once it is reviewed and approved, an approval email will be sent to you, and only then can the changes be implemented.
This requirement pertains to all applications, whether approved as Exempt, Expedited or Full Board. However, please note that changes that only impact the Project Roster (personnel changes), do not require the submission of the Amendment request form. Email the irb@ucr.edu directly with these changes.
-
When should I close out my IRB application?
It is the responsibility of the Principal Investigator to comply with continuing review requirements and submit required documentation in a timely manner in order to ensure that there will be no interruption in the research process. The Principal Investigator must ensure that their protocol does not meet or exceed the expiration date that has been initially approved.
For applicable studies, the Office of Research Integrity sends an expiration notification to the principal investigator 60 days prior to study expiration. However, the Principal Investigator will have ultimate responsibility for ansuring a timely submission of their protocol information. The required documentation for renewal must be completed and submitted to the ORI 30 days in advance of the next IRB meeting date to account for a timely pre-review of submitted materials. -
What is HIPAA and what do I need to do about it?
Anyone conducting research dealing with HIPAA also needs to complete the HIPAA tutorial. You will need to register in order to complete the tutorial.
When research involves the use or disclosure of PHI by entities subject to the regulations, the rules will apply. Researchers have legitimate needs to use, access, and disclose PHI to carry out a wide range of health research studies. In most instances, the Privacy Rule requires an authorization from the individual or a waiver of authorization from an IRB or Privacy Board before a covered entity can access, use or disclose PHI for research purposes. In general, there are two types of human research that would involve PHI:- Studies involving review of medical records as a source of research information.
- Studies that create new medical information because a health care service is being performed as part of the research.
The UCR IRB will approve either:
- A full waiver of authorization to conduct all the research activities described in the research proposal; or
- A partial waiver of authorization for specific research actives such as recruitment.
In most instances, a full waiver of authorization is granted only when there is no opportunity for the researcher to obtain authorization from the individual. Partial waivers of authorization are often granted to allow researchers to access the EMR to identify potential research participants.
-
Can I conduct human subjects research using prisoners as participants?
Yes; however, in order to conduct research with prisoners, researchers must adhere to additional regulations beyond the basic requirements for research with human subjects. Research with prisoners is governed by federal regulations that classify prisoners as a “vulnerable population” (45 CFR 46 Subpart C) because their ability to make an informed and voluntary decision to participate in research is compromised by their incarceration.
Certain types of research with prisoners are permissible but often require review and approval from several agencies including the prison facility. In the state of California, research is governed by the Research Review Process for the California Department of Corrections and Rehabilitation (CDCR). Additionally, the state of California has a penal code (3502a) prohibition on any biomedical research conducted on prisoners in the state. For this reason, the UCR IRB will not approve prisoners to be involved in any biomedical research studies. Biomedical research is defined by CA law as, “research relating to or involving biological, medical or physical science.” Authorization from the DHHS OHRP must be obtained prior to the initiation of any federally funded research involving prisoners.
Researchers are strongly encouraged to contact the IRB during the design of any research studies that may involve prisoners.
Definition of Prisoner
A prisoner, as defined by Federal Regulation (45 CFR 46.303 (c)) is any individual involuntarily confined or detained in a penal institution, including:- individuals sentenced to such an institution under a criminal or civil statute;
- individuals detained in other facilities by virtue of statutes or commitment procedures which provide alternatives to criminal prosecution or incarceration in a penal institution and;
- individuals detained pending arraignment, trial, or sentencing
Common examples of the application of the regulatory definition of prisoner are as follows:
- Individuals who are detained in a residential facility for court-ordered substance abuse treatment as a form of sentencing or alternative to incarceration are prisoners
- Individuals with psychiatric illnesses who have been committed involuntarily to an institution as an alternative to a criminal prosecution or incarceration are prisoners
- Parolees who are detained in a treatment center as a condition of parole are prisoners
Common examples of individuals who are not defined as prisoners according to the regulations:
- Individuals who are receiving non-residential court-ordered substance abuse treatment and are residing in the community;
- Individuals who have been voluntarily admitted to an institution for treatment of a psychiatric illness, or who have been civilly committed to non-penal institution because of their illness makes them a danger to themselves or others;
- Individuals living in the community and sentenced to community-supervised monitoring, including parolees, are not prisoners;
- Probationers and individuals wearing monitoring devices are generally not considered to be prisoners; however, situations of this kind frequently require an analysis of the particular circumstances of the planned subject population. Institutions may consult with OHRP when questions arise about research involving these populations.
Types of Permissible Research with Prisoners
On a case by case basis, the IRB must decide which one of the categories listed below best represents the proposed research:- §46.306(a)(2)(i): A study of the possible causes, effects, and processes of incarceration, and of criminal behavior, provided that the study presents no more than minimal risk and no more than inconvenience to the participants;
- §46.306(a)(2)(ii): A study of prisons as institutional structures or of prisoners as incarcerated persons, provided that the study presents no more than minimal risk and no more than inconvenience to participants;
- §46.306(a)(2)(iii): Research on conditions particularly affecting prisoners as a class (e.g., vaccine trials and other research on hepatitis which is much more prevalent in prisons than elsewhere; and research on social and psychological problems such as alcoholism, drug addictions, and sexual assaults);
- §46.306(a)(2)(iv): Research on practices, both innovative and accepted, which have the intent and reasonable probability of improving the health or well-being of the participant.
Federally funded research falling in categories iii and iv may only proceed after the HHS Secretary has consulted with appropriate experts, including experts in penology, medicine, and ethics, and has published notice in the Federal Register of his or her intent to approve the research.
When a Participant Becomes Incarcerated during a Study
If an ongoing study requires a visit with a participant who has become incarcerated during the course of the research, the researcher must cease all contact with the participant during his/her incarceration. Any data collected prior to the incarceration may still be used; however, new data may not be collected. If a study necessitates visiting the participant while they are incarcerated, the researcher must amend their IRB application to include prisoners as part of their participant population. The amendment will then be reviewed by the IRB according to the additional required regulations. -
I am a non-UC researcher at another campus who would like to access UCR participants, what do I need to do?
UCR does have a process that allows non-UC PIs to request approval to use UCR’s students, faculty and/or staff. The first step is to complete the UCR Administrative Review for Human Research Studies Being Conducted by Non-UCR Principal Investigators Accessing UCR Facilities, Patients or Personnel and submit that along with your approved IRB application (and its relevant materials [consent/survey/flyer/ad, etc.]), mention a UCR faculty member who’s assisting you (if applicable), and verification of human subjects training program to IRB@ucr.edu.
-
I want to start a drug trial, device trial or am not sure if my research with a drug or newly developed device falls under the purview of FDA regulated or clinical trials. Can you help me?
Yes. Depending on the type of research you are conducting and the type of product (if any) that has been developed, your research with any new App, drug, device, software, etc. may be subject to additional regulations with additional regulatory agencies (e.g. FDA, DoD). Please contact the ORI at IRB@ucr.edu to begin the application process to determine which federal regulations may apply to your research.
-
What is a “waiver” of informed consent and how do I apply for one?
A waiver of informed consent could: 1) alter some or all of the required elements of informed consent or 2) completely waive the requirement to obtain informed consent. The IRB may approve a consent procedure which does not include or alters some or all of the required elements of informed consent provided all of the following have been determined to be true by the IRB:
- The research involves no more than minimal risk
- The waiver of informed consent will not adversely affect the rights and welfare of the subjects
- It is not practicable to conduct the research without the waiver or alteration
- If the research involves using identifiable private information or identifiable biospecimens, the research could not be practicably carried out without using such information or biospecimens in an identifiable format.
- Whenever appropriate, participants will be provided with additional pertinent information after their participation.
Examples of types of studies in which some or all elements of consent have been waived include retrospective chart reviews, studies of existing specimens, ethnographic research, studies that require deception or passive (opt-out) consent. Also keep in mind that waiver of informed consent is where some or all elements of consent is removed. This is fundamentally different than a waiver of documentation of informed consent (see FAQ #19) where only the signature of the participant on the inform consent document is waived. I.e. only survey forms where a signed document would be difficult to obtain.
-
What is a waiver of documentation of informed consent?
A waiver of documentation of informed consent alters the informed consent process by eliminating the requirement for research participants to sign the informed consent form. This means that researchers are still required to provide participants with the required informed consent information, but a signature is not obtained. Investigators requesting this waiver will be required to include in their IRB application the consent information that will be provided to the participants and details on how consent will be documented in lieu of the signed form (e.g., investigators will include in their research notes that participant ‘X’ provided consent).
The IRB may approve a consent procedure which waives the documentation of informed consent provided the IRB finds the research to meet the following:- The only record linking the participant and the research would be the consent document;
- The principal risk would be potential harm resulting from a breach of confidentiality; and
- Additionally, each participant will be asked whether the participant wants documentation linking the participant with the research, and the participant’s wishes will govern;
OR - The research is minimal risk and
- Involves no procedures that usually require written consent;
OR - The participants or legally authorized representatives are members of a distinct cultural group or community in which signing forms is not the norm;
- The research presents no more than minimal risk of harm to participants; and
- There is an appropriate alternative mechanism for documenting that informed consent was obtained.
Examples of studies where the documentation requirement have been waived include studies on sensitive topics such as violence or illegal activities and studies where participants may not be interacting directly with the investigators such as telephone or web-based surveys.
-
What are Short Forms?
A short is a written document stating that the elements of informed consent required by 45 CFR 46.117 have been presented to and understood by the subject or the subject's legally authorized representative.
A short form may be used when the majority of subjects in a study are English speakers, but there are a portion of the subjects who will not be able to understand the consent form written in English. A short form can be used in these instances to obtain consent ensuring equal access for potential participants.
If the majority of the anticipated subjects to be enrolled do not speak English or will be unable to understand the consent form written in English, the consent form must be translated into a language understandable to the subjects. When the person obtaining consent is assisted by a translator, the translator may serve as a witness. The subject must be given copies of the short form document and the summary.
A short form may be used in conjunction with an oral presentation of the consent information required by 45 CFR 46.117. A summary of what will be said to the subject or representative must be approved by the IRB and then presented orally to the subject or representative in front of a witness. A copy of the short form and a copy of the summary must be given to the subject or representative.
Required signatures with short forms- Short Form - Subject/Representative and the Witness
- Copy of Summary - Witness and Person obtaining consent
In a research application, the UCR IRB must receive justification for the use of short forms, a summary of what will be presented to the subject or representative and the text of your short form.
-
I’m conducting a project which uses an Oral History methodology, do I need IRB review?
It depends. Determining whether a project using oral history methodologies requires IRB review involves evaluating whether the investigator is engaged in the creation of "generalizable knowledge".
Federal regulations define “research” as “a systematic investigation, including research development, testing, and evaluation, designed to develop or contribute to generalizable knowledge.” The 2018 revision of the Common Rule (45 CFR 46) included a provision to clarify that certain activities are excluded from the federal “research” definition. These activities include:
Scholarly and journalistic activities (e.g., oral history, journalism, biography, literary criticism, legal research, and historical scholarship), including the collection and use of information, that focus directly on the specific individuals about whom the information is collected (45 CFR 46.102(l)(1)).
Oral history activities intended to create a record of specific historical events and/or provide an accurate and evidence-based portrayal of the individuals involved are not meant to contribute to generalizable knowledge and hence do not require IRB review.
If, however, the project uses oral history activities designed with the intent to collect information and draw general conclusions, inform policy or test a hypothesis, then this project would require IRB review. Researchers must complete and submit the General IRB Application Form and Project Roster to irb@ucr.edu for review.
Examples:- An oral history video recording of interviews with World War II survivors is created for viewing in the WWII Museum. The purpose is to create a historical record of specific personal events and experiences related to the War and provide a venue for survivors to tell their stories. The creation of the videotape is not intended to prove a hypothesis, inform policy, or draw conclusions. Therefore, this project would not constitute research requiring IRB review.
- Oral history interviews are conducted with surviving Gulf War veterans to document their experiences in order to draw conclusions about those experiences, inform policy, and generalize findings. This project would constitute research requiring IRB review.
Remember, you can always contact the IRB for a consultation to discuss your project by emailing irb@ucr.edu.
-
When do I need to give the Experimental Subject's Bill of Rights form to my research participants?
California law, under Health & Safety Code §24172, requires all investigators doing a "medical experiment" to offer their subjects a copy of the "Experimental Subject's Bill of Rights." Failure to do so may result in civil or criminal penalties. To ensure compliance with California law, investigators should document that each subject received the form using one of the methods described below.
A "medical experiment" is defined as:
The severance or penetration or damaging of tissues of a human subject, or the use of a drug or device as defined in section 109920 of 109925 (of the Health and Safety Code), electromagnetic radiation, heat or cold, or a biological substance or organism, in or upon a human subject in the practice or research of medicine in a manner not reasonably related to maintaining or improving the health of such subject or otherwise directly benefiting such subject. The investigational use of a drug or device as provided in Sections 111590 and 111595. Withholding medical treatment from a human subject for any purpose other than maintenance or improvement of the health of the subject.
UC has interpreted this definition to include almost all studies involving biomedical procedures, placebo controls, innovative therapy and/or normal volunteer subjects.
For these types of studies, you must give a copy of the UCR Experimental Subject's Bill of Rights to subjects in a language in which the subject is fluent, along with a copy of the study's consent document and possibly the HIPAA Authorization Form should the study include collection of protected health information (PHI).
For non-biomedical studies, the IRB may recommend use of the Experimental Subject's Bill of Rights, though it is not required by law.
How Should Researchers Document Dispensation of the Form?
Subjects do not sign the Experimental Subject's Bill of Rights, but you should document that each subject received the form by one of the following methods:- Keep a copy of the Bill of Rights with the subject’s initials in his/her study file;
- Write a note on the consent form confirming that the subject received the Bill of Rights;
- Write a note in the subject’s research record confirming that the subject received the Bill of Rights; OR
- Keep a copy of the Bill of Rights in the subject’s research file with the original signed consent form (and HIPAA authorization form, if applicable).
Please do NOT include the Bill of Rights in the appendices of your IRB application.
-
What is the revised Common Rule?
The U.S. Federal Policy for the Protection of Human Subjects, also known as the ‘Common Rule’, is the baseline standard of research ethics by which any research in the U.S. is held; nearly all U.S. academic institutions hold their researchers to these standards regardless of funding. UC applies the Common Rule standards to all human subjects research studies.
The U.S. Department of Health and Human Services and fifteen other Federal Departments and Agencies have issued final revisions the Common Rule. A final rule was published in the Federal Register (FR) on January 19, 2017, and was amended to delay the effective and compliance dates on January 22, 2018, and June 19, 2018. The revised Common Rule will be effective on January 21, 2019.
This revised rule strengthens protections for people who volunteer to participate in research, while ensuring that the oversight committee does not add inappropriate administrative burdens, particularly to low-risk research. It also allows more flexibility in keeping with today’s dynamic research environment.
The Revised Common Rule does not apply to FDA-regulated and Department of Justice-funded human subjects research studies.
-
What are the major changes of the Revised Common Rule?
Major Regulation Changes
- Continuing Review - No longer required for most minimal risk research, including studies where the only remaining activity is the analysis of identifiable data/biospecimens or activity to obtain follow-up clinical data. However, there are exceptions to this rule.
- Exemptions – There are new exemption categories and clarification of existing categories. Some exemptions may require * “limited IRB review”. The UC system has already been exercising the new exempt category for certain research.
- * Limited IRB review is a process that is required only for certain exemptions and does not require an IRB to consider all of the IRB approval criteria in §46.111. In limited IRB review, the IRB must determine that certain conditions, which are specified in the regulations, are met. Limited IRB review may be done via the expedited review mechanism, that is, by the Chair or an experienced IRB member designated by the Chair (although it can also be conducted by the full IRB). Continuing review is not required.
- Informed Consent Document - A new ‘key information’ section will be required for those detailed consents, such as those required for Clinical Trials. This section must present a summary of all pertinent information to participants in the study. Additionally, this re-arrangement of content must be designed to facilitate a potential subject's decision to participate or not.
- Single IRB-of-Record (sIRB) - IRB oversight for most federally-funded collaborative research projects located in the U.S. will be required to use a single IRB (commercial, academic, or hospital-based) starting January 20, 2020. This may mean you will need to use either the Reliance Registry or SMART IRB for central IRB review for federally funded, multi-site studies. For use of sIRB, please contact ORI in advance of your proposal.
Please be advised that UC will not be using the Broad Consent exemptions (categories 7 & 8) at this time. These two exemptions will not be implemented because UC as an institution cannot track consent as is required for these categories.
Minor Regulation Changes
- Addition of 4 categories of scholarly and journalistic fields that do not constitute research in and of themselves. However, it only excludes certain activities, the new common rule (post-2018) does not excuse the entire academic field
- Changes to how the IRB maintains membership, operations and record keeping, including appropriate access to meeting space and staff to support IRB review and recordkeeping duties
- A requirement for the establishment of written procedures for conducting IRB reviews and for ensuring prompt reporting of changes to the IRB
- Pregnant women or handicapped or physically disabled individuals are no longer included as examples of populations that are potentially vulnerable to coercion or undue influence.
- The term “individuals with impaired decision-making ability” now replaces the term “mentally disabled persons.”
-
Does the new Common Rule allow researchers to self-identify their projects as exempt?
No. This requirement remains unchanged from the current Common Rule to the Revised Common Rule. An assessment by an IRB member will still be needed in order to determine if a project meets guidelines for exemption.
-
How will the Revised Common Rule affect the review of IRB applications?
- New
IRB Applications submitted after the implementation date (January 21, 2019) will be subject to the Revised Common Rule requirements. - Pending (In Review/Not yet approved)
Applications submitted prior to the implementation date but are still in the review process as of January 21, 2019, will be subject to the revised Common Rule requirements. As such, additional revisions may be requested to comply with the revised regulations. - Existing applications
All projects reviewed and approved prior to the implementation date remain under the old rule. These projects retain their existing level of review and all other IRB requirements, including continuing review requirements. Existing applications that would like to convert to the revised requirements will be provided case-by-case information on transitioning to the new rules (if applicable).
- New
-
How will the Revised Common Rule affect the Continuing Renewal review process?
For new studies approved on or after January 21, 2019, continuing review will no longer be required for most minimal risk studies. For new studies determined to qualify for exempt or limited IRB review under the revised Common Rule, continuing review requirements do not apply. For new studies determined to qualify for expedited review under revised Common Rule, the regulations now stipulate that continuing review is not required unless justified by the IRB. Therefore, most expedited studies will not require continuing review. Justifications for continuing review might include:
- The project involves additional regulatory oversight.
- Study procedures or risks indicate greater oversight is necessary.
- Other research specific considerations.
Additionally, continuing review is not required for research that has progressed to the point that it involves only one or both of the following, which are part of the IRB-approved study:
- data analysis, including analysis of identifiable private information or identifiable biospecimens, or
- accessing follow-up clinical data from procedures that subjects would undergo as part of clinical care.
Although continuing review will not be required for some eligible Expedited studies, Principal Investigators are still responsible for the following:
- Being familiar with and adhering to the Principal Investigator Responsibilities outlined in the IRB General Application form found in the IRB section of the Forms page
- Submitting amendments for study changes
- Reporting events and unanticipated problems, and
- Closing the study once it ends
-
How will the revised Common Rule affect Existing and Ongoing Research?
The revised Common Rule requires existing research (approved or determined exempt prior to January 21, 2019) to remain compliant with the previous regulations. Therefore, for existing studies, researchers do not need to take any action at this time beyond maintaining active IRB approval. Maintaining IRB approval includes submitting for continuing review (unless previously granted exemption). Existing research can be transitioned to operate under the revised Common Rule only after being reviewed and determined to comply with the revised Common Rule. To be brought into compliance with the revised regulations, existing studies may require substantial revisions.
The revised Common Rule offers flexibility to allow existing studies to remain under the previous regulations, existing studies are not required to comply with the revised Common Rule. Studies cannot pick and choose components between the two sets of regulations. In order to transition to the revised Common Rule, a study must be determined to satisfy ALL criteria under those new (aka revised) regulations. Once a study transitions to the revised Common Rule, it cannot transition back to the old Rule. The only exception would be if a study approved under the revised Common Rule obtains funding by the Department of Justice or becomes subject to FDA oversight. In this instance, the study would then need to transition to the old rule.
Existing studies requesting to transition to the revised Common Rule will be assessed for transition readiness on a case-by-case basis.
-
How will the revised Common Rule affect the Informed Consent Process and documentation?
The revised Common Rule requirements implement changes to the content, organization, and presentation of information to facilitate a participant’s comprehension and decision about whether to take part in research. These changes include the use of a Key Information section prior to the detailed (i.e., full) consent form. The key information section should be a concise and focused presentation of the key details that is most likely to assist in understanding the reasons why one might or might not want to participate in the research. Following the key information page, the detailed consent form will follow all previous requirements but may include some of the new required elements listed below.
New required element of informed consent for studies involving collection of identifiable private information or identifiable biospecimens. One of the following statements must be in the informed consent:- A statement that the identifiers might be removed from the information, and after such removal, the information could be used for future research studies or distributed to another investigator for future research without additional informed consent; OR
- A statement that the subject’s information or specimens, even if identifiers are removed, will not be used or distributed for future research
New additional elements of informed consent will be required for applicable research studies. These additional elements are:
- A statement that the subject’s biospecimens (even if identifiers are removed) may be used for commercial profit and whether the subject will or will not share in this commercial profit;
- A statement regarding whether clinically relevant research results; including individual research results, will be disclosed to subjects, and if so, under what conditions; and
- For research involving biospecimens, whether the research will (if known) or might include whole genome sequencing
-
Whom do I contact if I have questions about the revised Common Rule changes?
The Office of Research Integrity is available to answer questions regarding the Revised Common Rule changes and can be contacted via email at irb@ucr.edu.
There are also several online resources available as well:
-
What are some of the items NOT in the final revised Common Rule?
- Self-exemption determinations by researchers;
- Federally issued tools such as consent templates, checklists;
- IRB review and approval requirement for de-identified bio-specimens;
- A Requirement to include non-required elements of consent in an appendix
-
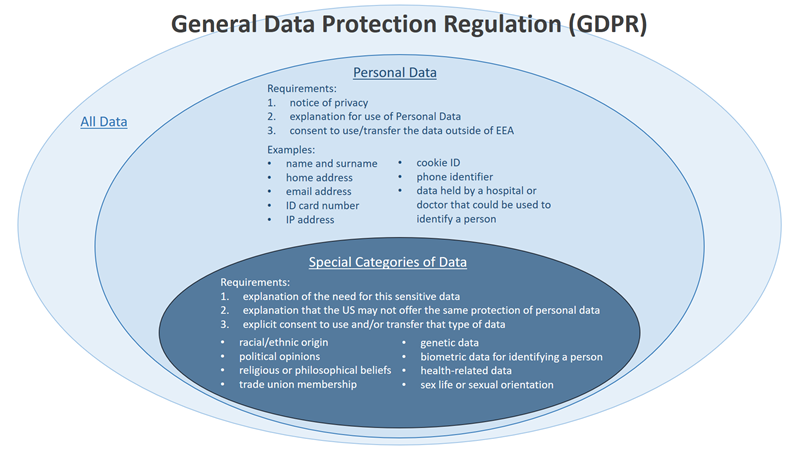
What is GDPR?
The General Data Protection Regulation (GDPR) is designed to protect the personal data of individuals who are located in the European Economic Area (EEA). Personal data is any data that can be used to identify an individual. The EEA includes The European Union (EU) plus the United Kingdom (UK) as well as Iceland, Lichtenstein, and Norway. GDPR is intended to be an overarching privacy regulation for all EU Member States and replaces prior EU privacy regulations. In contrast, the U.S. has no single data privacy law with an equally broad application; rather, U.S. privacy laws regulate certain sectors, such as health care (the Health Insurance Portability and Accountability Act (HIPAA)) and student records (the Family Education Rights and Privacy Act (FERPA)). GDPR also addresses the transfer of personal data outside the EEA.
-
What does GDPR do?
GDPR expands privacy rights for individuals located in the EEA regardless of citizenship. Specifically, with respect to certain activities of U.S. organizations, it guarantees certain “fundamental rights” to individuals in the EEA. These include:
- Right to information: Data subjects have the right to be informed about the collection, processing and use of personal data;
- Right of access: Data subjects have the right to obtain copies of their personal data being processed;
- Right to rectification: Data subjects have the right to ensure correction of any inaccurate personal data about them;
- Right to erasure (“right to be forgotten”): Data subjects have the right to request erasure of their data;
- Right to restriction of processing: Data subjects have the right to restrict certain processing activities relating to their data;
- Right to data portability: Data subjects have the to right to receive personal data which they have provided and the right to have that personal data transferred to another party;
- Right to object: Data subjects have the right to object to the processing of their data; and
- Automated individual decision making, including profiling: Data subjects have the right to contest solely automated processing, or profiling, activities relating to their data.
GDPR impacts data pertaining to these individuals even when the data is located in other countries, regardless of the citizenship of the individuals. Specifically, GDPR establishes a framework for safeguarding how personal data is used, such as:
- Ensuring that the data is transferred, processed, stored and eventually disposed of using appropriate technical safeguards,
- Limiting the use/processing of the data to purposes that comply with GDPR requirements (e.g., managing the academic records of UC students studying in the EEA as part of Education Abroad),
- Requiring suppliers who access the data in the course of providing a service to agree to certain protections and safeguards of the personal data.
-
What is the territorial scope of GDPR?
GDPR applies to organizations located within the EEA that process personal data about any person anywhere in the world. It also applies to organizations outside of the EEA if they offer goods or services to, or monitor the behavior of, EEA data subjects, regardless of the organization’s location or whether the data subject is an EEA citizen. With respect to UCR research activies, GDPR requirements apply when:
- A UCR researcher monitors or performs research on individuals in the EEA (e.g., research on the behavior of EEA citizens in virtual world environments),
- A UCR researcher engages in research using personal data while that researcher is located in Europe (e.g., collaborating with a European research center in Europe), or
- Offering goods or services to individuals in the EEA (e.g., providing gift cards for participation in research or interviewing and hiring research fellows from the EEA).
-
What is Personal Data and how do GDPR’s guidelines for protecting Personal Data differ from those of the United States?
According to GDPR, ‘personal data’ means ANY information relating to an identified or identifiable natural person (‘data subject’). An identifiable natural person is one who can be identified by either:
- Directly identifying information (e.g., name, surname, phone numbers, etc.), or
- Pseudonymous* data (i.e., coded data) or non-directly identifying information (e.g., behaviors or beliefs), which does not allow the direct identification of users but allows the singling out of individual behaviors (e.g., to have a webpage serve a customized ad).
Personal data is more broadly defined under GDPR than the types of data protected by any one U.S. federal or state privacy law. Thus, if you are collecting or using data from individuals located in a country located in the EEA, in most cases GDPR will apply.
*Although Pseudonymous data is still subject to GDPR, pseudonymization is an appropriate way to safeguard Personal Data. In fact, GDPR requires that Personal Data be pseudonymized if the purpose of the research can be accomplished by using pseudonymized data.
-
How does GDPR define Special Categories of Personal Data?
GDPR identifies a subset of personal data, called Special Categories, that require additional protections. Special Categories are defined as personal data that reveals any of the following types of information about an individual:
- Racial or ethnic origin
- Political opinions
- Religious or philosophical beliefs
- Trade union membership
- Genetic data
- Biometric data for the purpose of uniquely identifying a natural person
- Health condition, sex life and/or sexual orientation
If any Special Categories of Personal Data of an individual in the EEA is collected, used, or accessed for research purposes, the researcher must:
- Explain to participants how and why their data will be used, and
- Acquire explicit consent from each participant for using their special categories of personal data for each specific purpose. If the researcher has not collected the information him or herself, the researcher must ensure that the data subject has been informed of, and has consented to, the use of their special categories of personal data for the purposes intended by the researcher.
-
What types of research activities are subject to GDPR requirements?
GDPR is applicable to a broad range of research activities. For example, GDPR may apply:
- When UCR acts as a sponsor of research occurring in EEA member states; and
- When UCR acts as a core data facility or lead site for a multi-national research study with EEA-based sites;
Where UCR is not obtaining any personal data from individuals located in the EEA, GDPR does not apply. This means that where UCR is only enrolling subjects locally (in the United States), and is merely providing personal data to a sponsor located in the EEA, GDPR does not apply. GDPR does apply to the sponsor in this case, but not to UCR.
However, GDPR does apply to UCR where UCR is receiving personal data of individuals located in the EEA from an EEA sponsor or where UCR is recruiting individuals located in the EEA to participate in studies being conducted in the United States.
Research studies that collect data online from EEA residents/visitors may also be subject to GDPR. GDPR has no “grandfather provision” or exemptions allowing use of data collected without GDPR-compliant consent. Therefore, it is unlawful to process any EEA data collected prior to May 25, 2018, unless it can be shown that each participant received GDPR-compliant notices and provided GDPR-compliant consent.
The only case in which data about individuals is not subject to GDPR is when the data is anonymized. Anonymization is a high standard under GDPR: all direct and indirect identifiers of an individual must be removed, and the researcher must implement safeguards that ensure that the data can never be re-identified. For data to be truly anonymized under GDPR, the anonymization must be irreversible.
-
What does GDPR require from those who perform research on or collect data from EEA residents/visitors?
Entities whose research activities meet at least one of the GDPR criteria described in the response to the “What types of research activities …?” question above are required to provide notice of privacy to each individual whose personal data is collected. This must include an explanation of the reason for collecting personal data as well as the individual’s rights regarding accessing/withdrawing the data and lodging complaints. UCR has created this Statement of Privacy Practices and Procedures for providing the institution’s privacy information to research participants.
When UCR researchers collect personal data from individuals in the EEA and intend to access the data in the United States, or transfer the data outside of the EEA, the researcher must also obtain consent from the individuals to transfer the data back to the U.S.. Thus, in most, if not all scenarios in which a researcher is collecting personal data, consent to transfer the data to the U.S. will be required. In addition to obtaining consent of the individual, GDPR requires that invidiuals also be informed that the United States does not protect personal data in the same manner as the EEA does.
If any of the data collected meet the definition of Special Categories of Personal Data, then GDPR requires an explanation of the need to collect sensitive data. In addition, each individual must give explicit consent to use that type of data. If the Special Categories of Personal Data will be transported outside of the EEA, individuals must give explicit consent for that as well.
This template can be adapted to provide GDPR-compliant Notice and Informed Consent for the participants in your research.
-
What is an IACUC?
The Institutional Animal Care and Use Committee (IACUC), is established by federal mandate at institutions that use live, vertebrate animals for research, teaching, and testing activities. The IACUC oversees and evaluates all aspects of the institution's animal care and use program.
-
How do I know if I need IACUC approval for the animal research I would like to begin?
If you intend to use vertebrate animals for research, testing, or educational activities at UCR or an affiliate, you need IACUC approval. This includes field research involving the capture or handling of wild animals. Contact the IACUC Office (iacuc@ucr.edu) to determine if the activities require IACUC review and approval.
-
How do I submit an application to receive approval to perform animal research?
The IACUC reviews and approves applications through our cloud-based application software, Kuali. A step-by-step training guide is provided to help with your submission. Besides scheduled training, the IACUC Office is available during Kuali Assistance Office Hours on the third Wednesday of the month, from 1:00 pm - 2:00 pm.
Meeting ID: 917 8850 3062
Passcode: 495899 -
Once submitted, how long does it take an animal use protocol (AUP) to be approved?
The time it takes from submission to approval depends on many factors, including the type of procedures performed on animals (e.g., surgery vs. behavioral tests), the type of changes requested when submitting an amendment, and how the researcher answers the questions on the AUP. The turnaround also depends on the type of submission. New AUPs can take up to 90 days from submission to approval. An amendment to an already approved AUP can take up to 3 weeks from submission to approval. A three-year renewal with no changes can take up to 30 days, while a renewal with changes takes up to 60 days.
-
Are there any training requirements that must be completed before I can access animals?
Yes. In addition to enrolling in UCR's Occupational Health Program, all animal researchers must complete the training described in the Training Checklist.
-
I have completed all the required training but still can’t access the animals in the vivarium. How can I get access to a UCR vivarium?
Depending on where your animals are housed, an orientation to the vivarium is required before access is granted. Once you have completed the IACUC training requirements, the IACUC Office should provide information on completing the vivarium tour. If you have already completed the training but have not received instructions on how to complete the vivarium tour, please contact IACUC Training for assistance (iacuctraining@ucr.edu).
-
I just submitted a new AUP. Now what?
The submission is being pre-reviewed by the IACUC Administration Team. Once that pre-review is completed, the Attending Veterinarian will complete their pre-review. The purpose of these pre-reviews is to address any administrative or veterinary concerns. After the pre-review, the AUP will be returned to the principal investigator (PI) to address the comments, make necessary updates to the AUP, and resubmit. Once resubmitted, the AUP will be assigned for IACUC Committee review.
-
What are the different types of IACUC review?
As described in Section V. of Policy #529-224, AUPs that must be reviewed by the IACUC are reviewed via Designated Member Review or Full Committee Review. The IACUC Office can complete an administrative review for minor updates.
-
When does the committee meet to review submitted AUPs?
The committee meets once a month to review AUPs that are assigned FCR. A calendar of review dates and submission deadlines is available for you to review under the section Submit Your AUP.
-
I just submitted an amendment to add a procedure to my already approved AUP. Can I start performing the new procedure since the amendment has been submitted?
No. No procedures on animals can be performed until the amendment has been reviewed and approved by the IACUC. No exceptions.
-
How often do I need to renew my AUP?
The PHS Policy requires a complete review of each current AUP every three years. Federal regulations do not allow extensions, and all vertebrate animal activities must stop until the AUP has been reviewed and approved.
-
I am getting an error message in Kuali and having an issue submitting.
An error message usually indicates that the application has not been completed and that the required questions have not been addressed. The IACUC Office is available to help; please reach out to us at iacuc@ucr.edu with any concerns or questions.
- What is a Research-Related Conflict of Interest (COI)?
-
What is the Conflict of Interest (COI) committee?
The Conflict of Interest Committee, is responsible for the review and assessment of all financial disclosures related to research projects at UC Riverside, as well as determining any actions required to ensure that real or perceived financial research-related conflicts of interest are managed, reduced or eliminated.
- How are Research-Related COIs and Conflict of Commitment (COC) related?
-
What is a Financial Conflict of Interest (FCOI)?
In accordance with the University of California Policy on Disclosure of Financial Interests and Management of Conflicts of Interest Related to Public Health Services Sponsored Awards for Research (42 C.F. Part 50, Subpart F and 45 C.F.R., Part 94): The Principal Investigator and all other UCR investigators must disclose their personal significant financial interests (and those of their spouse/registered domestic partner and/or dependent children) related to their institutional responsibilities. This includes the Principal Investigator, Co-Investigators, Senior and Key Personnel, and any other individual who is responsible for the design, conduct, or reporting of research funded by PHS or an agency or organization that follows PHS disclosure requirements (for example, American Heart Association, American Cancer Society, etc.). This Form is to be completed by the Principal Investigator, and those Project Personnel designated by the Principal Investigator as an Investigator, as defined by the 2011 Revised PHS Regulations.
-
How are FCOI’s managed?
After PRO determines that a financial interest rises to the level of a financial research-related conflict of interest, PRO will create a management plan recommendation. The PRO Committee works with the Investigator to create the management plan that strives to be the simplest effective means of managing the conflict. Each management plan is situation-specific which is why it requires cooperation between the Investigator and PRO.
The PRO Committee submits a recommendation to the VC-RED. The VC-RED will determine whether the management plan is sufficient and if so, the plan is presented to the Investigator. The Investigator will be required to review the plan and sign that they agree to comply and implement the plan. -
What are some general principles to keep in mind when starting a company?
- Keep your company activities separate and distinct from UC faculty activities;
- Expect your company to be treated exactly like any other company (the fact it’s partially owned by a UC faculty member does not give it any special privileges.);
- File a disclosure to evaluate potential conflicts and mitigate risk if necessary;
- Not all research-related conflicts are impermissible and most are manageable;
- When human subjects are involved, there will be a higher level of scrutiny;
- Case-by-case analysis and management is crucial;
- When in doubt, disclose to PRO.
-
Can I start a company?
Yes. UCR's research-related conflict of interest and conflict of commitment policies do not prohibit investigators from starting their own company, however depending on the type of research the investigator conducts, there may be conflict of interest disclosure requirements and approvals required. Please contact PRO for more information.
In the case individuals are looking for information related to these types of start-ups and entrepreneurial activities at UCR, they can be directed to RED’s 'Entrepreneurial Proof of Concept and Innovation Center’ (EPIC) website and staff. -
Can I serve on a board of advisors/directors?
Yes, UCR's research-related conflict of interest policy does not prohibit investigators from serving on a Board of Directors, Advisors, or similar. If this position also involves other financial interests such as income or equity interests, the investigator may have additional COI disclosure requirements. If PRO determines that these interests could potentially be a conflict of interest with the research, the Committee will require the investigator to take steps to manage, reduce or eliminate the conflict before the research can proceed. Investigators who are contemplating serving on a board should contact PRO as soon as possible to discuss possible conflict of interest issues related to their research.
Service on a Board of Directors carries with it legal fiduciary responsibility, but generally not management responsibility and hence, is generally permissible. The investigator’s primary commitment is to the University and service on a Board of Directors should not interfere with his/her primary obligations as a faculty member or university employee. For more information on COC, see this page provided by the Office of Academic Personnel. -
Can I serve on a scientific advisory board?
Yes, serving on a Scientific Advisory Board is permitted because such positions do not carry, nor are they perceived to carry, management responsibility. The investigator, however, should recuse himself/herself from any discussion or decision to fund his/her own research. If this position also involves other financial interests such as income or equity interests, the investigator will have additional COI disclosure requirements.
-
Can I participate in research sponsored by a company that licensed technology I invented at UCR?
Yes. However, your participation in the research could be considered a potential research-related conflict of interest if you have a financial or equity interest in the sponsor. If you have financial interests in the sponsor, you may need to disclose and have the paperwork reviewed by PRO.
For a detailed summary of the thresholds, please see the State Law column in the UCR COI Disclosure Chart.
If you do not have a financial or equity interest in the sponsor, you will not need to be reviewed by PRO.
If you have any questions, please reach out and contact us so we may schedule a consultation. -
What are some examples of mitigation or management plans to address COIs?
Here are some examples of how to address potential research-related conflicts of interest:
- Public disclosure of significant financial interests;
- Training on research-related conflicts of interest for all personnel involved in the research;
- Monitoring of research by independent reviewers;
- Double-blind study and/or randomized study
- Modification of the research plan to ensure objectivity;
- Have non-conflicted investigators collect data and perform data analyses;
- Remove oneself as the Principal Investigator;
- Disqualification from participation in all or a portion of the research;
- Divestiture of significant financial interests;
- Severance of relationships that create actual or potential conflicts
-
I serve as consultant to a company and want to test a product from that company. Does having non-conflicted investigators and the study being blinded assist in mitigating the conflict?
Yes, having a non-conflicted investigator and blinding the study will help mitigate the conflict; however, PRO will still need to review all the factors to determine if that is necessary and/or sufficient. As part of the PRO review, these are some but not all of the other factors the PRO will consider:
- Who has supervisory authority of all research personnel involved in the study?
- Are there any research personnel under the supervision of the conflicted individual?
- Is (Are) the non-conflicted investigator(s) junior to the conflicted individual?
- Are students involved and if so, who is supervising them?
- Who’s doing the data analysis, collection, and reporting?
- Are there Human Participants involved?
Depending on the level of risk created from the financial interest and how the study is designed, PRO may require additional measures to protect the objectivity of the research.
-
May I own stock in a publicly traded company and serve as an investigator on a study for that company’s products?
You could serve as the investigator on a study, however depending on who the sponsor of the study is and the percentage and/or the value of the stock, you may be required to disclose and go through a PRO review. For a more detailed summary of the thresholds, please see the UCR COI Disclosure Chart. If you have this type of situation, please contact us so we may schedule a consultation.
-
Through the university, I am an inventor on a patent. May I serve as an investigator testing its effectiveness?
Potentially yes, but it depends on the sponsor of the research. It also depends on whether you have a financial interest in the sponsor or one related to the research project, and how you would manage that financial interest. If you have a financial interest in the sponsor or one related to the research, your situation may need to be reviewed by PRO. If you have this type of situation, please contact us so we may schedule a consultation.
-
I’m a co-founder in a company that would like to provide funds to my lab at UCR through an industry-sponsored research agreement, am I allowed to do that? What should I be concerned about?
It depends. One thing you will need to be concerned about is making sure you do not represent both the university and the company in the industry-sponsored research negotiations or any business transactions. For example, we would probably recommend 1) your company hire a lawyer to represent the company in business transactions with university, or 2) your company include (or add) an individual without any university affiliation so when UC Riverside is working with the company, that person is the company representative.
However, please note that your participation in the research could be considered a potential research-related conflict of interest if you have a financial interest in the company and are the Principal Investigator or Co-Principal Investigator. If you, the Principal Investigator or Co-Principal Investigator of the study, have financial interests in the company, you will need to disclose and have the paperwork reviewed by PRO. If you have any questions, please contact us so we may schedule a consultation. -
I’m a consultant to a company that would like to provide funds to my lab at UCR through an industry-sponsored research agreement, am I allowed to do that? What should I be concerned about?
Yes, it is allowable for the company to provide funds to your lab but that could potentially trigger a COI review. If you have a financial interest in the sponsor and you are the Principal Investigator (or Co-Principal Investigator), your disclosures may need to be reviewed and approved by PRO. If you have this type of situation, please contact us so we may schedule a consultation.
-
I own stock (have equity) in a company that would like to provide funds to my lab at UCR through an industry sponsored research agreement, am I allowed to do that? What should I be concerned about?
Yes, it is allowable for you to own stock or have equity in the company that would like to provide funds to your lab. However, if you do have a financial interest in the company and you are the Principal Investigator (or Co-Principal Investigator), your disclosures may need to be reviewed and approved by PRO. If you have this type of situation, please contact us so we may schedule a consultation.
-
I have a positive disclosure for my project that will be sent to PRO for review. When will I receive the results of the review, so my study funds can be released?
Depending on the nature of the disclosure, administrative review of submitted forms can take approximately 1 – 2 weeks after all supplemental forms have been submitted. For disclosures requiring full committee review, completion of the process will take approximately 4 – 6 weeks. See the PRO website for additional information on when the PRO committee meets.
-
When not reporting COIs, what are some of the potential risks?
If you have any concerns regarding COI issues, we strongly encourage you to contact our office immediately. There are significant risks involved for not reporting:
- Compromise of scientific integrity (objectivity of the research);
- Misuse of University facilities;
- Improper direction of student’s or University employee’s work;
- Unbalanced allocation of faculty member’s time and effort;
- Failure to recognize the University’s right to intellectual property and related financial interests;
- Improper channeling of research funds;
- Inappropriate delay or restriction on publications;
- Appearance of impropriety
-
What is Research Misconduct (RM)?
Research Misconduct (RM) means fabrication, falsification, or plagiarism in proposing, performing or reviewing research, or in reporting research results. Research Misconduct does not include honest errors, differences of opinion or authorship disputes.
- Fabrication is making up data or results and recording or reporting them
- Falsification is manipulating research materials, equipment or processes, or changing or omitting data or results such that the research is not accurately represented in the research record
- Plagiarism is appropriating another person’s ideas, processes, results or words without giving appropriate credit
-
What is UCR’s policy regarding Research Misconduct?
UCR takes RM allegations seriously. You can review Policy #529-900: Policy and Procedures for Responding to Allegations of Research Misconduct at https://research.ucr.edu/about/policies-ucr.aspx?k=31.
-
As a researcher, what are some evidence-based best practices that are available to me in leading and managing a lab?
It is crucial that University researchers maintain safe and professional working environments in their laboratories. The NIH-funded P.I. Program has created this checklist to assist with Lab Leadership and Management. Please review the additional RM resources available on the Resources page of our website.
UCR also has a Research Ethics Education Program which can assist UCR researchers with meeting responsible conduct of research training requirements.
The following are additional strategies that researchers can use to avoid being involved in allegations of research misconduct.- Discuss authorship with all research collaborators at the outset of a project so everyone involved understands who will be listed as an author, and the expectations regarding the use of the data by those involved in the research.
- Monitor the research in which you are involved –- inform your staff, students and collaborators, that you will verify data collection, entry and reporting. Ask questions about questionable results.
- Set reasonable expectations about the time it will take to collect the necessary data.
- Maintain thorough and complete research records.
- Respect the research process.
- Do not stray from the protocol without obtaining the necessary approvals.
- Communicate any actual or perceived problems with the research. Most research misconduct allegations are the product of communication difficulties between researchers.
- Carefully and accurately report the research. Be specific about methods and procedures used and the data obtained.
- Thoroughly review all papers where you are listed as an author.
- Do not give or agree to guest-author status.
- Promote research integrity –- teach the responsible conduct of research in your courses and labs and encourage attendance at Responsible Conduct of Research programs sponsored by the Office of Research Compliance and Integrity.
-
I suspect that Research Misconduct is occurring in my lab, but I’m not sure if I should report it. Where can I go for advice?
UCR’s primary RM contact, including questions about whether an activity constitutes RM, is the Assistant Research Integrity Officer (ARIO), Gillian Wilson at gillianw@ucr.edu. UCR’s Research Integrity Officer is Rodolfo Torres, Vice Chancellor for Research and Economic Development
For confidential counseling or confidential guidance on the topic, you may consult the UCR’s Office of the Ombuds at 951-827-3213.
If you prefer to report to an independent agency outside of UCR, visit the EthicsPoint website or call them at 800-403-4744. This independent agency is contracted to provide RM reporting services for the University of California. -
Who is involved in Research Misconduct review?
There are four primary parties involved in RM reviews. There may be additional parties involved, but the essential parties are:
- Complainant – the person who makes an allegation of RM
- Respondent – the subject of the RM allegation
- ARIO/RIO - Assistant Research Integrity Officer/Research Integrity Officer – the person(s) who execute(s) a fair, impartial and competent review of allegations of research misconduct
- Witness(es) – Any person(s) pertinent to the RM inquiry or investigation
-
Are the names of Research Misconduct review participants kept confidential?
Confidentiality is maintained throughout the investigation of the alleged research misconduct to the greatest extent possible and consistent with federal and state laws. However, under California law, information pertaining to a misconduct case can become publicly available (upon request) at the end of the investigation.
-
To whom can I report research misconduct?
If you suspect someone is engaged in research misconduct, please report it to:
- UCR’s Assistant Research Integrity Officer (ARIO), Gillian Wilson at gillianw@ucr.edu
- UCR’s Research Integrity Officer (RIO), Rodolfo Torres, Vice Chancellor for Research and Economic Development
- Your Department Chair or PI
- Your Unit Dean/Director
- The University of California’s independent reporting agency, EthicsPoint at 800-403-4744
-
What should I do if I am accused of Research Misconduct?
- University investigators have an obligation to investigate RM accusations. Be honest and cooperative throughout the investigation.
- Avoid doing anything that may be interpreted as retaliation. The University has a policy that strictly prohibits retaliating against whistleblowers.
- To discuss the matter confidentially, you can contact UCR’s Office of the Ombuds.
- Keep thorough records of your research and training in the lab – especially lab notebooks - and all related communications. If the investigation fails to conclude that research misconduct has occurred, be sure to save written confirmation of the outcome.
-
What are the main steps in the Research Misconduct review process?
A misconduct allegation leads to a review of the associated evidence. This review consists of three subsequent steps. After each step, responsible University officials have the opportunity to determine if there is sufficient cause continue the RM review.
Assessment - The Research Integrity Officer (RIO) reviews the allegation and conduct an assessment to determine whether:- it matches the definition of research misconduct and
- it is credible and specific enough so that evidence of the misconduct can be identified. (often, faculty members or staff who have expertise in the subject area assist with the assessment)
Inquiry – If there is sufficient evidence to support 1 and 2 above, then the RIO appoints a panel of faculty and/or staff members to review the alleged misconduct. This panel determines whether there is significant substance to the allegation to warrant a formal Investigation.
Investigation – A separate panel examines the evidence in depth and interviews all relevant witnesses to determine whether misconduct has been committed, by whom, and to what extent. The panel makes recommendations regarding whether the respondent acted intentionally, knowingly or recklessly. The panel’s conclusion is then transmitted to the RIO, who decides whether to accept it. -
What are the possible outcomes of the Research Misconduct review process?
A full Research Misconduct review process may be extensive and involve several steps. However, there are points throughout the process where a University Officials may decide to discontinue the review and close the case. This diagram shows some the possible outcomes for RM reviews.