About the IACUC
UCR recognizes the scientific and ethical responsibility for the humane care and use of animals involved in research and education and enjoins all individuals involved to the highest standards of care and consideration.
UCR has established an Institutional Animal Care and Use Committee (IACUC) to function as the review body responsible for approval and oversight of activities involving the use of vertebrate animals at UCR in accordance with federal requirements, including the Animal Welfare Act and PHS Policy. The Committee, appointed by the Vice Chancellor for Research and Economic Development, is qualified through the experience and expertise of its members to oversee the institution's animal program, facilities, and procedures in coordination with the UCR Campus Veterinarian. Additionally, the IACUC is committed to promoting open and cooperative relationships with investigators and educating the UCR community concerning the ethical and regulatory standards for the humane care of animals.
IACUC Policies, Guidance, and Information for Faculty
- Policies and Guidance
- IACUC and AUP Information for UCR Faculty (related, grant, housing and inspection information)
- Frequently Asked Questions (FAQs)

Step 1: Determine if you need to submit an Animal Use Protocol (AUP)
An AUP is required for all research and teaching activities involving live vertebrate animals at UCR or sponsored by UCR. This includes:
- All vertebrate animal activities (i.e., receiving, housing, maintaining, field studies, teaching uses, and lab experiments, including "gentling" treatments and non-invasive studies)
- Vertebrate animal activities performed at other institutions under contract from UCR (e.g., contracting to have antibodies generated or new mouse strains developed; or when a UCR investigator is lead PI on a grant with subcontracts to other institutions).

Step 2: Training
-
Occupational Health Reviews
Occupational Health Reviews
Occupational Health Reviews must be completed before handling vertebrate animals in research and teaching.
No one may enter the vivarium without the following:
- Clearance notification from the IACUC office and
- Completion of vivarium orientation with vivarium staff
This applies to all animal users (PIs, lab managers, graduate students, etc.) working with laboratory animals. These safety considerations are in place to avoid exposure to (a) zoonotic diseases (infectious agents shared by humans and animals), (b) allergies to laboratory animals, particularly rodents, and (c) bites, scratches, and other injuries. All animal users are required to participate in the Occupational Health Surveillance System (OHSS).
Enrollment in the OHSS is described in the "Training Required Before Handling Vertebrate Animals in Research and Teaching" section.
-
Training Required Before Handling Vertebrate Animals in Research and Teaching
Training Required Before Handling Vertebrate Animals in Research and Teaching
Federal regulations require that all individuals handling vertebrate animals receive proper training, including, but not limited to, federal law regarding animal use, species-specific handling and experiment-specific procedures, zoonotic diseases, methods that minimize the number of animals required to obtain valid results, and training in the specific procedures necessary for experiments. UCR uses an online training system for general training and has species-specific videos available. The IACUC Office and The Office of the Campus Veterinarian are available to provide hands-on training in various procedures. Training in project-specific procedures is the responsibility of the AUP PI and must be documented.
The steps needed to enroll in the OHSS and to complete IACUC required training are available in the Lab Training Checklist.

Step 3: Submit your AUP
AUPs may only be submitted by individuals meeting PI eligibility as defined by UCR Policy #527-3. Academic coordinators planning to use vertebrate animals in teaching may request an exception. Contact iacuc@ucr.edu to request an exception.
Access Kuali to create and submit a protocol.
-
IACUC Levels of Review
IACUC Levels of Review
IACUC Levels of Review Full Committee Review (FCR)
The entire IACUC reviews protocols during a regularly scheduled meeting. Designated Member Review (DMR)
Protocols are reviewed by a designated IACUC member. Regardless of review level, all new AUPs may be subject to additional review by EH&S and the Office of the Campus Veterinarian to review potential hazardous materials and to ensure animal welfare is upheld. Protocols that request conducting surgeries outside of the vivarium require scientific justification and are subject to semi-annual inspections by the IACUC as mandated by federal regulations.
-
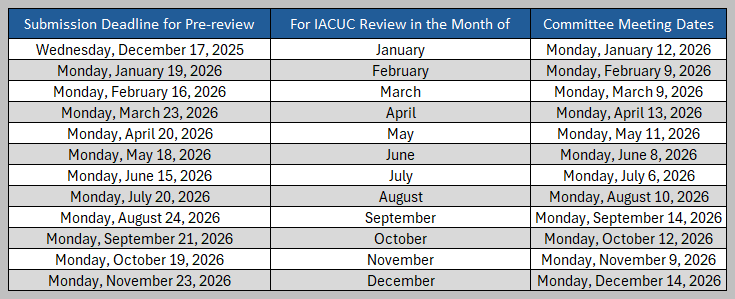
Submission Deadlines for Full AUPs and Amendments
Submission Deadlines for Full AUPs and Amendments
-
Things all Animal Users/Handlers at UCR Should Know
Things all Animal Users/Handlers at UCR Should Know
- All individuals must complete the training and occupational health requirements prior to handling live vertebrate animals in research and teaching
- PIs are responsible for ensuring each laboratory member receives hands-on training for any procedure they perform. Training may be performed by the PI, a designated and experienced laboratory member, an experienced colleague, or as arranged with the Office of the Campus Veterinarian. Laboratory members should not attempt procedures they have not received training for or do not yet feel confident in performing.
- Training performed in the laboratory must be documented.
- AUPs and implementation by investigators must minimize potential pain and distress by:
- Ensuring laboratory members have been trained appropriately, are competent in the procedure, and are familiar with determining if the animal is experiencing pain or distress.
- All laboratory members are familiar with and have access to the latest approved AUP.
- All procedures, including the use of anesthesia, analgesia, and animal monitoring, are performed according to the AUP.
- Collaborations involving the treatment of animals under more than one AUP must be approved on an AUP.
- The Campus Veterinarian must be contacted when adverse or unexpected events occur regarding the use of animals. An unexpected event is any occurrence that requires veterinary care or causes unintentional pain, distress, or mortality.
- IACUC must be aware of and have approved the sequence of all treatments a single animal will be exposed to undergo.
- Euthanasia must be performed as approved in the AUP. Euthanasia procedures must adhere to the AVMA guidelines for the euthanasia of animals.
- Handle materials safely - PIs must ensure that lab members receive appropriate safety training (both the courses offered by EH&S and in-lab training for lab-specific procedures).
- PIs and laboratory staff listed on an approved AUP should be familiar with UCR IACUC policies and guidelines.
- Animals housed in the UCR vivarium must be ordered through the Office of the Campus Veterinarian unless otherwise authorized by the Campus Veterinarian.
- No procedure shall be performed on any animal unless the procedure has been reviewed and approved by the IACUC.