About IRB-Clin
The Institutional Review Boards (IRBs) are committed to following the federal regulations to protect the rights and welfare of human subjects involved in research conducted under the auspices of the University of California, Riverside (UCR). UCR upholds the highest standards in the ethical conduct of research, including the protection of human participants, while enabling its faculty, staff and students to conduct research in a timely and efficient manner.
UCR currently has two IRBs, IRB-SB (socio-behavioral) and IRB-Clin (clinical-biomedical). IRB-Clin functions as the review body responsible for the approval and oversight of clinical-biomedical research at UCR. Drug or devices trials (Phase I-IV) need to be submitted for review through the WIRB-Copernicus Group after contacting Research Compliance (ORC). For all Clinical Trials, researchers must first contact ORC prior to submitting an application.
The primary mission of the IRBs is to facilitate those objectives by reviewing, approving, modifying or disapproving research applications submitted by UCR researchers, and in some cases, non-UCR researchers. The IRB process is based on rules and regulations for federally funded research, primarily the provisions of Protection of Human Subjects in the Code of Federal Regulations (45 CFR 46 and 21 CFR 56), and supporting documents such as the Belmont Report. UCR’s IRBs strive to create an on-campus culture of respect for, and awareness of, the rights and welfare of human research participants, while advancing knowledge and facilitating the highest quality research.
Do I have to submit an IRB application?
If your study meets the definition of human subjects research, you must submit an IRB application Research Compliance (ORC). All human subjects research must receive prior approval from one of the IRBs.
If you are unsure, you can fill out and submit the Determination of Activity form found in the IRB section of the ORC Forms Page. This form will assist the ORC in determining whether your activity meets the definition of ‘research involving human subjects’ and requires IRB review/approval.
Systematic Investigation |
|
 |
|
Generalizable Knowledge |
|
 |
|
Human Subject |
|
 |
|
Human Subjects Research |
|
Do I have to submit to the IRB-Clin (as opposed to the IRB-SB)?
IRB-Clin reviews studies that involve:
- Use of a biological substance or organism, in or upon a human subject in the practice or research of medicine in a manner not reasonably related to maintaining or improving the health of the subject or otherwise directly benefiting the subject.
- The investigational use of a drug or device
- Withholding medical treatment from a human subject for any purpose other than maintenance or improvement of the health of the subject.
- Any of the following procedures being done for research with no reasonable expectation that patient care will be improved or maintained:
- Skin Penetration (including blood draw) or penetration of tissue inside an orifice (e.g., blood samples, biological samples or swabs, skin or tissue biopsies outside of normal shedding)
- Application of heat (e.g., exposing participants to heat exposure to determine heat stress, or tolerance of high temperatures)
- Application of cold (e.g., cold or freezing temperature exposure to determine tolerance or biological effects)
- Application of electric current & electromagnetic fields (e.g., medical device use such as EKG, EEG or MRI)
- If treatment is being withheld (Including nutritional supplements, pharmaceuticals and studies with treatment arms where cohort will not receive the investigational agent)
For help in determining which IRB will be reviewing your application, please refer to the comparison table below. Depending on the complexity and risk levels associated with your study, the ORC will make the final determination regarding the IRB that a study application is relevant to.
|
Applications that involve: |
IRB-Clin |
|
|
Focus groups, surveys and interviews are conducted with or without participants' identifying information |
✔ |
|
|
Focus groups, surveys and interviews are conducted and include blood draws, application of heat, cold or electric current |
|
✔ |
|
Focus groups, surveys and interviews are conducted and participants are asked to provide biological samples or biopsies |
|
✔ |
|
Participants will be monitored by EEG, EKG, MRI, X-Ray and/or other medical devices |
|
✔ |
|
Participants will be subjected to mild electric shock, as part of study interactions |
|
✔ |
|
Research involving materials (e.g., data, documents, records) that have been collected or will be collected solely for research purposes |
✔ |
|
|
Surveys asking about participant health, drug use, or other medical information |
✔ |
|
|
Research asking how participants feel about an investigational drug or device or dietary supplement |
✔ |
|
|
Research asking participants to use an investigational drug or device or dietary supplement* |
|
✔ |
|
The severance or penetration or damaging of tissues of a human subject |
|
✔ |
*Note: For Clinical Trials, please contact Research Compliance at IRB@ucr.edu directly to arrange
an external review prior to submitting an application.
How to fill out and submit an application
To fill out an application for the IRB-Clin, please complete the Application Form for Use of Human Participants and Project Roster Form (both on ORC's Resources Page). Once the form has been completed and additional documents have been attached, please email us the application at IRB@ucr.edu.
Common mistakes that may delay your review and approval include not providing enough information pertaining to your study or not attaching all relevant documents requested/listed in the application form. (This includes all assessments or information given to participants.)
Categories of Review
The Level of Review
If your study needs IRB review, the next step is for ORC to identify the level of review required – Full Board review, Expedited review or Exempt certification.
The level of review usually reflects the level of risk to the subject. The risk level is compared to “minimal risk” research as defined by the federal regulations:
The definition of Minimal Risk states: the probability and magnitude of harm or discomfort anticipated in the research are not greater than those ordinarily encountered in daily life or during the performance of routine physical or psychological examinations or tests [45, CFR 46, 102(j)].
The UCR Minimal Risk Tip Sheet (available on the ORC Resources page) is a tool developed by ORC to help the researcher determine what level of risk their study may fall under. You can use this form to help you make this determination as part of your research on human subjects study application.
Full Board |
IRB applications that involve more than minimal risk or do not meet the criteria for Exempt or Expedited. They are reviewed at a monthly convened IRB meeting. Examples of applications that may require full board review are studies using invasive procedures and research with investigational test articles. |
|
|---|---|---|
Expedited |
Minimal risk studies meeting specific criteria as outlined in 45 CFR 46.110. Applications are generally reviewed by a delegate process of IRB review. |
|
Exempt |
Revised Common RuleStudies originally approved on or after January 21, 2019, meeting specific criteria as outlined in 45 CFR 46.104(d) Exempt categories. Please note this excludes FDA regulated and DOJ funded studies. |
Common RuleStudies meeting specific criteria as outlined in 45 CFR 46.101(b). |
Funding agencies do not allow investigators to make exempt determinations on their own, nor does the University. Instead, they must submit the study to the IRB, which will make this determination. The IRB will NOT certify as exempt the following types of research at UCR:
- FDA regulated
- More than minimal risk
- Involves inpatients as subjects
- Involves the intentional use of prisoners as subjects
- Some uses of deception
IRB application Forms (“Where do I find these?”)
IRB applications can be found on ORC's Forms Page. Submit completed forms to: IRB@ucr.edu. Additional information such as the Informed Consent Guide can be found in the IRB section of the ORC Resources page.
Timelines
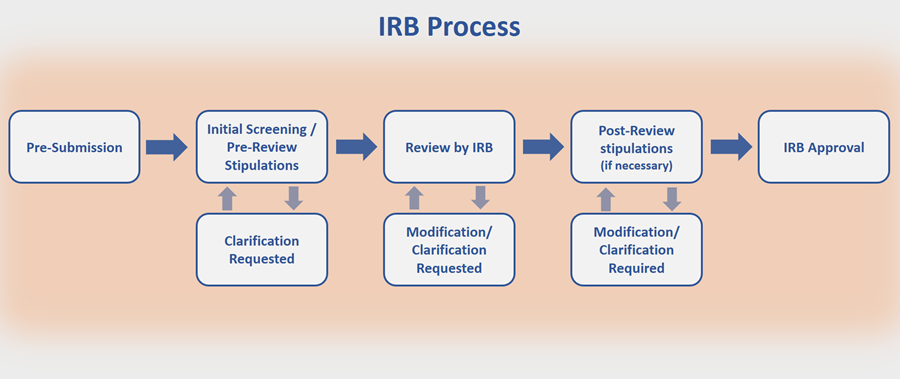
The IRB utilizes an initial pre-review screening process whereby each application is reviewed for completeness and compliance. During this process, researchers may be asked to make changes to their submissions before being reviewed by the IRB (pre-review). Once the researchers address the pre-review comments, the IRB then reviews the revised applications. IRB reviewers may also ask for changes or clarifications, which IRB analysts will communicate to the research team after IRB review (post-review). See “Top Reasons an Application is Delayed” for more information.
As detailed in the above “Categories of Review" section, there are three types of IRB review, as
determined by the ORI. Depending on the level of review, the following timelines apply. These review times
are averages. Depending on the complexity of the application and workload in the office, additional
time may be necessary to conduct appropriate reviews.
|
Level of Review |
Average Review Times |
|
Full Board |
For Full Board review, IRB comments will be forwarded to you within two weeks following the regularly-scheduled monthly meeting. Please see below the schedule of IRB-SB meetings & submission deadlines. |
|
Expedited |
Submissions for expedited review are currently taking eight to nine weeks to be processed and reviewed. Rolling submissions are accepted. |
|
Exempt |
Requests for exemption determinations review are currently taking eight to nine weeks to complete. Rolling submissions are accepted. Researchers must still submit an IRB application to get an 'exempt' determination. |
Schedule of IRB-Clin Meetings & Submission Deadlines
for Full Board Reviews
For other application types that ORI has determined full board review is not required, the duration of the review and approval process will vary based on your study and may take several weeks. Applications should always be sent in well ahead of any research to be conducted.
~ important points to keep in mind ~
The IRB-Clin normally meets on the third Wednesday of each month. In order for an application to be placed on the agenda for a meeting, the researcher should plan to submit the application well in advance of the anticipated study start and adequately address any issues raised during the review process.
Depending on the complexity and risk levels associated with your study, the Office of Research Integrity will categorize your application submission for review. Applications will be reviewed on a first-come, first-served basis. This includes study amendment applications and continuing renewals. Therefore, the ORI highly recommends accurate and timely submission of study applications and attachments to account for this review process.
Investigators may receive requests from ORI to make changes to submission during the pre-review screening process and/or after IRB Member/Board review. These comments must be addressed prior to IRB approval and a deadline for response will be provided. Once satisfactory responses are received, the ORI moves the applications to the next step in the review process.
IRB Consultations & Contact Information
University of California, Riverside
Office of Research Integrity
900 University Ave.
213 University Office Building
Riverside, CA 92521
Phone: 951-827-4802
The Office of Research Integrity offers IRB consultations for UCR investigators and research staff who are preparing IRB submissions (new studies and amendments) or preparing responses to review comments. Please email us at IRB@ucr.edu to set up an appointment to speak with an ORI representative.
Education & Training
- Required Human Subjects Protection Training - All investigators and staff conducting human subjects research are required to complete the Collaborative Institutional Training Intiiative (CITI) online courses.
- For IRB-Clin specific training, select the courses that specifically pertain to “Human Subjects Research for Biomedical Research Investigators".
- IRB approval cannot be issued until the Lead Researcher and advisor/supervisor (if applicable) have completed the required ethics training.
- The Lead Researcher is responsible for ensuring the completion of Human Subjects Protection Training for all research personnel (including staff and students) involved in the research.
You can find information about WIRB and Clinical Trials here.
Where can I find additional information?
For information on HIPAA requirements in research, medical device risk assessment and additional information for Clinical & Biomedical research, please visit the IRB section of the ORI Resources Page.