This page reviews the principles of Responsible Conduct of Research (RCR) and the use of CITI courses to satisfy RCR training requirements.
Please note: this is not the same as the Human Subjects Research (HSR) training required for IRB applications. For instructions regarding HSR training, visit CITI Program Training for IRB Requirements.
Commonly asked Questions
-
What is Responsible Conduct of Research (RCR)?
Responsible Conduct of Research (RCR) involves the awareness and application of established professional norms and ethical principles in the performance of all activities related to scientific research. Projects funded by NSF, NIFA, and NIH have different requirements regarding training that vary by funder and role on the study.
There is currently one Responsible Conduct of Research (RCR) course in CITI. However, learners are encouraged to review relevant optional courses.
The basic RCR course contains the following modules:- Introduction to RCR (RCR-Basic) (ID 17009)
- Authorship (RCR-Basic) (ID 16597)
- Collaborative Research (RCR-Basic) (ID 16598)
- Conflicts of Interest and Commitment (RCR-Basic) (ID 16599)
- Research Misconduct (RCR-Basic) (ID 16604)
- Peer Review (RCR-Basic) (ID 16603)
- Data Management (RCR-Basic) (ID 20896)
- Mentoring and Healthy Research Environments (RCR-Basic) (ID 20983)
- Presentation of Research Findings (ID 19355)
- Plagiarism (RCR-Basic) (ID 15156)
Optional modules:
- Human subjects RCR
- Environmental and Social Dimensions of Engineering
- Animals RCR
- Financial Responsibility (ID 16601)
- Reproducibility of Research Results (ID 17756)
- Communicating with the Public (ID 19270)
- Do I have to complete RCR training?
-
What is UCR's plan to meet the Federal Requirements Federal RCR training requirements?
The Institutional Plan to Meet the Federal Requirements for Training and Oversight in the Responsible Conduct of Research outlines UCR’s plan to meet the Federal RCR requirements and the responsibilities of individuals and departments at UCR.
-
What are the training requirements for specific funding agencies?
What are the training requirements for specific funding agencies?
Projects funded by NSF and NIFA have different requirements regarding training in RCR.
NIH RCR training requirements cannot be met by completing CITI courses since they must be face-to-face or in-person training.
Federal funding agencies that have requirements for RCR training
Federal Funder Minimal Requirement for RCR Target Audience
(who must do the training)CITI RCR Training
QualifiesNotes NSF Online or face-to-face training Faculty, Senior personnel, Undergraduates,
Graduate students,
Postdoctoral researchers✔
Includes faculty training NIFA Online or face-to-face training Faculty,
Program Directors,
Undergraduate students,
Postdoctoral researchers,
any staff✔
Includes faculty training NIH * Face-to-face training/interaction Trainees, Fellows,
Scholars, ParticipantsX
Must be completed at least once during each career state and at least once every 4 years * For NIH, the requirements for such training apply to all NIH Institutional Research Training Grants, Individual Fellowship Awards, Career Development Awards (Institutional and Individual), Research Education Grants, and Dissertation Research Grants. The programs are listed as:
D43, D71, F05, F30, F31, F32, F33, F34, F37, F38, K01, K02, K05, K07, K08, K12, K18, K22, K23, K24, K25, K26, K30, K99/R00, KL1, KL2, R25, R36, T15, T32, T34, T35, T36, T37, T90/R90, TL1, TU2, and U2R.
-
Where do I obtain the required training?
- NSF or NIFA funding: use CITI's online RCR training to fulfill the RCR training requirements.
- NIH funding: face-to-face RCR training is required. Contact the Director of the Research Ethics Education Program, Dr. Dena Plemmons at dena.plemmons@ucr.edu or 951-827-4312
- NSF or NIFA funding: use CITI's online RCR training to fulfill the RCR training requirements.
-
Where can I find more information regarding RCR?
Several resources are available for UCR researchers. The Office of Research Compliance (ORC) provides RCR information in the RCR section of the Resources Page. Also, many professional societies and governmental licensing authorities have adopted policies or best practices that might be usefully considered.
- What units on Campus are involved in RCR training?
-
Whom can I contact?
If you need to arrange for in-person RCR training, or are interested in templates for tracking training, contact the Director of the Research Ethics Education Program, Dr. Dena Plemmons at dena.plemmons@ucr.edu or 951-827-4312.
For information regarding online training and requirements, contact RED's AVC Charles Greer at charles.greer@ucr.edu.
-
Transferring CITI Course Credits
If you completed a CITI course or some of its modules previously at another institution, you can transfer the credits to your UCR-affiliated account:
Instructions to transfer credits to your UCR-affiliated account
-
Step-by-step instructions to enroll in CITI Program's Online RCR Training
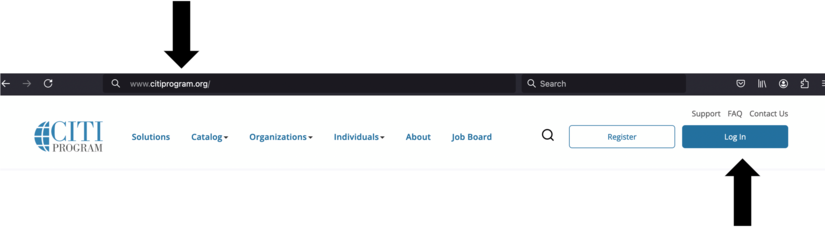
- Choose the Log In button at https://citiprogram.org
-
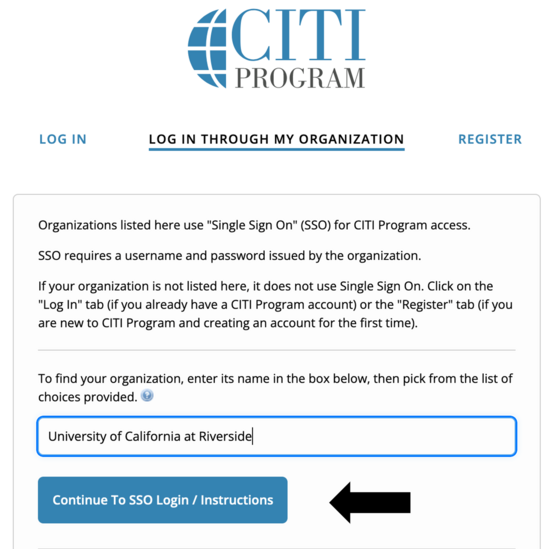
- Start typing "Riverside" -- it will bring up University of California at Riverside, which you'll then choose
-
-
- Choose either “I already have a CITI program account” [go to step 10 if this is true for you] or “I don’t have a CITI program account and I need to create one”
- Complete registration
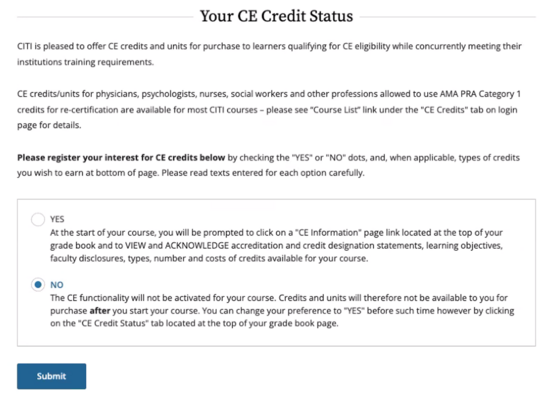
- This will always be a NO choice for these modules
-
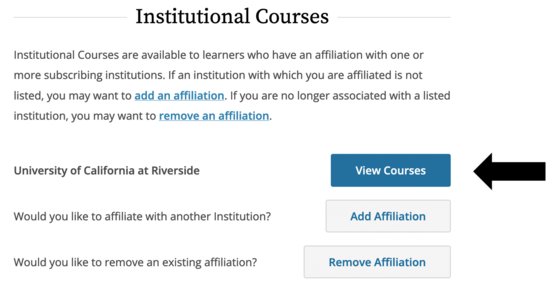
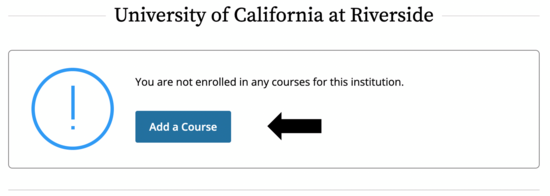
- It may be the case that you are not enrolled for any courses through CITI, in which case you’ll see this screen. If you are already enrolled in other courses, they will show up here.
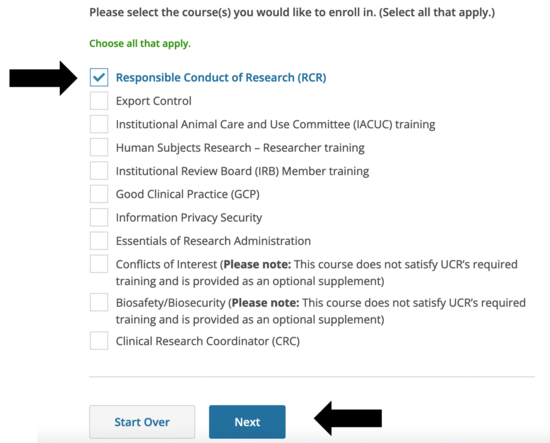
- You will need to enroll in the RCR course
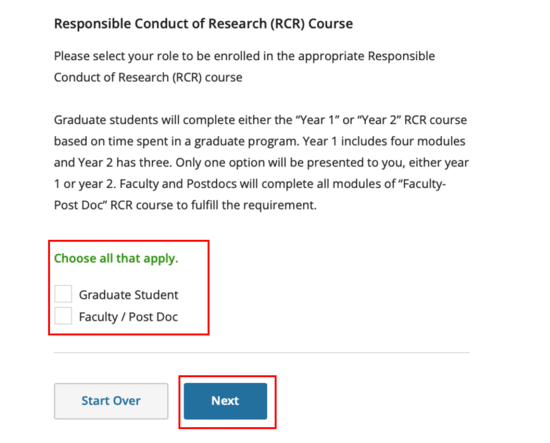
All personnel on federal awards, including students, should select the Faculty/Post Doc option -
-
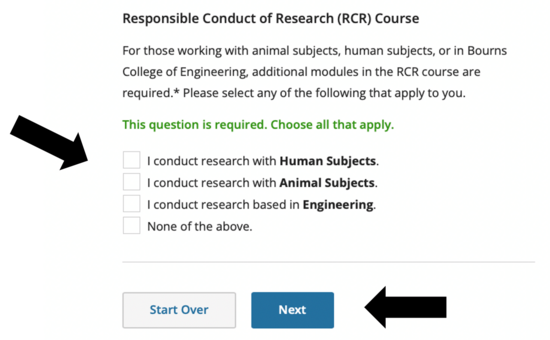
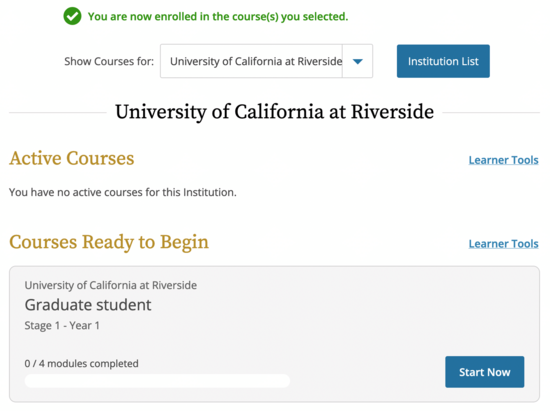
- If you checked “none of the above” on the previous screen, this is what you’ll see for the RCR course. If you are already enrolled in other CITI courses, they will be here as well.
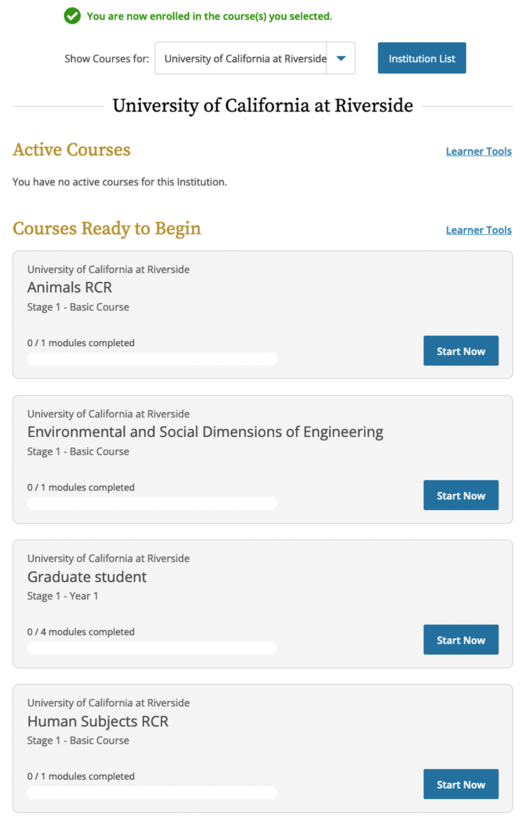
- If you checked human subjects, animal subjects and / or engineering on the previous screen, this is what you’ll see for the RCR course. If you are already enrolled in other CITI courses, they will be here as well.
- As you’ll discover, each module for the different topics includes a video for either life sciences, social/behavioral/educational/education sciences, or biomedical sciences. We do realize this does not represent all possible disciplines, and we encourage you to choose the one most closely aligned with your field/discipline.
- Choose the Log In button at https://citiprogram.org