About the IRB
The Institutional Review Board (IRB) is committed to following the federal regulations to protect the rights and welfare of human subjects involved in research conducted under the auspices of the University of California, Riverside (UCR). UCR upholds the highest standards in the ethical conduct of research, including the protection of human participants, while enabling its faculty, staff and students to conduct research in a timely and efficient manner.
UCR currently has one IRB which functions as the review body for the approval and oversight of all human subjects research at UCR. The primary mission of the IRB is to facilitate those objectives by reviewing, approving, modifying or disapproving research applications submitted by UCR researchers, and in some cases, non-UCR researchers. The IRB process is based on rules and regulations for federally funded research, primarily the provisions of Protection of Human Subjects in the Code of Federal Regulations (45 CFR 46), and supporting materials such as the Belmont Report. UCR’s IRB strives to create an on-campus culture of respect for, and awareness of, the rights and welfare of human research participants, while advancing knowledge and facilitating the highest quality research.

Determine if you need to submit an IRB protocol
If your study meets the definition of human subjects research, you must submit an IRB protocol to the IRB Office. All human subjects research must receive prior approval from the IRB.
If you are unsure, you can complete and submit a Not Human Subjects Research (NHSR) Determination (formerly Determination of Activity or DOA) through the online IRB protocol submission system, Kuali. This submission type will assist the ORI in determining whether your activity meets the definition of ‘research involving human subjects' and requires IRB review/approval. For new submissions, please access UCR's Kuali Protocol Page.
Systematic Investigation |
|
 |
|
Generalizable Knowledge |
|
 |
|
Human Subject |
|
 |
|
Human Subjects Research |
|

Determine the Review Level
If your study needs IRB review, the next step is to identify the level of review required:
- Full Board review
- Expedited review
- Exempt certification.
The level of review usually reflects the level of risk to the subject. The risk level is compared to "minimal risk" research as defined by federal regulations:
Definition of Minimal Risk
the probability and magnitude of harm or discomfort anticipated in the research are not greater than those ordinarily encountered in daily life or during the performance of routine physical or psychological examinations or tests [45, CFR 46, 102(j)].
The UCR Minimal Risk Tip Sheet is a tool developed by the ORC to help researchers determine what level of risk their studies may fall under. You can use this form to help you make this determination as part of your research on human subjects study application.
Full Board |
IRB applications that involve more than minimal risk or do not meet the criteria for Exempt or Expedited. They are reviewed at a monthly convened IRB meeting. Examples of applications that may require full board review are studies using invasive procedures and research with investigational test articles. |
|
|---|---|---|
Expedited |
Minimal risk studies meeting specific criteria as outlined in 45 CFR 46.110. Applications are generally reviewed by a delegate process of IRB review. |
|
Exempt |
Revised Common RuleStudies originally approved on or after January 21, 2019, meeting specific criteria as outlined in 45 CFR 46.104(d) Exempt categories. Please note this excludes FDA regulated and DOJ funded studies. |
Common RuleStudies meeting specific criteria as outlined in 45 CFR 46.101(b). |
Funding agencies do not allow investigators to make Exempt determinations on their own, nor does the University. Investigators must submit their studies to the IRB, which will make this determination. The IRB will NOT certify as exempt the following types of research at UCR:
- More than minimal risk
- Involves inpatients as subjects
- Involves the intentional use of prisoners as subjects
-
Some uses of deception

Complete IRB Application Forms
For new submissions, please access UCR's Kuali Protocol Page.
To submit a protocol for IRB review, please access the IRB protocol form through the Kuali system and select “IRB Human Subjects Review” as the submission type. Submission of the protocol occurs directly within the system.
For information regarding available training opportunities, please see below resources.
Kuali IRB Resources
Common mistakes that may delay your review and approval include not providing enough information pertaining to your study or not submitting all relevant documents requested/listed in the protocol form. This includes all assessments or information given to participants. Please see Reasons for IRB Delays below.
Timelines
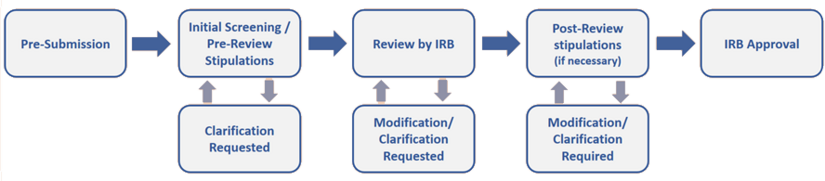
The IRB utilizes an initial pre-review screening process whereby each application is reviewed for completeness and compliance. During this process, researchers may be asked to make changes to their submissions before being reviewed by the IRB (pre-review). Once the researchers address pre-review comments, the IRB then reviews the revised application. IRB reviewer(s) may also ask for changes or clarifications, which they will communicate to the research team after IRB review (post-review).
-
Reasons for IRB Delays
Top Reasons for Delay
- Not following the instructions in the protocol
- Not providing required attachments (e.g., informed consent form, survey instruments, questionnaires, etc.)
- Including inconsistent information between the IRB protocol and the consent form
- Not providing consistent information on the handling and storage of sensitive and/or identifiable data
- Not providing sufficient detail on how the informed consent process will take place
- Not providing information that is requested after review
- Failing to provide letters of permission (access letters) from external sites involved in the research
- Not completing the Human Subjects Protection Course for all those involved in the research
- Incomplete Ancillary Review by Department Chair or designee
- Submitting the protocol before contacting the IRB office to discuss research ethics issues
For more information, contact irbsupport@ucr.edu.
IRB Process Flowchart
As detailed above in "Review Levels", there are three types of IRB review, as determined by the ORC. Depending on the level of review, the following timelines can apply. These review times are averages. Depending on the complexity of protocols and the workload in the office, additional time may be necessary to conduct appropriate reviews. Please note, these review times do not include the time the protocol is with the research team for revisions or time spent for ancillary reviews (i.e., required reviews by other offices such as ITS, FERPA, SOM Compliance, etc.).
|
Full Board |
For Full Board review, IRB comments will be forwarded to you within two weeks following the regularly-scheduled monthly meeting. Please see below the schedule of IRB meetings & submission deadlines. |
|
Expedited Exempt |
Submissions for Minimal Risk studies are currently taking approximately 8-9 weeks to be processed and reviewed. Rolling submissions ARE accepted. |
Schedule of IRB Meetings & Submission Deadlines for Full Board Review
Please submit by 3:00 pm. Dates subject to change.
| Submission Deadline | Meeting Date |
|---|---|
| October 20, 2025 | November 17, 2025 |
| November 17, 2025 | December 15, 2025 |
| December 22, 2025 | January 26, 2026 |
| January 26, 2026 | February 23, 2026 |
| February 17, 2026 | March 16, 2026 |
| March 16, 2026 | April 20, 2026 |
| April 20, 2026 | May 18, 2026 |
| May 18, 2026 | June 15, 2026 |
| No July Meeting | |
| July 20, 2026 | August 17, 2026 |
| August 17, 2026 | September 21, 2026 |
| September 21, 2026 | October 19, 2026 |
| October 19, 2026 | November 16, 2026 |
| November 14, 2026 | December 14, 2026 |
| December 14, 2026 | January 25, 2027 |
Please note: Submitting a Full Board protocol by the deadline does not guarantee placement on the IRB meeting agenda. Only complete and review-ready protocols will be placed on the agenda. If you have questions about the status of your submission, please contact the IRB office.
For other application types that the ORC has determined full board review is not required (i.e., minimal risk), the duration of the review and approval process will vary based on your study and may take several weeks. However, to facilitate review, please ensure that you address all requested revisions and return your application promptly to the IRB. Applications should be submitted well ahead of any research to be conducted.
Important points to keep in mind
- The IRB normally meets on the 3rd Monday of each month. For an application to be placed on the agenda for a meeting, researchers should plan to submit their applications well in advance of the anticipated meeting date and adequately address any issues raised during the pre-review process. Only protocols that are found to be complete/ready for review will be placed on the meeting agenda.
- Depending on the complexity and risk levels associated with the study, Research Compliance will categorize the application submission for review. Applications will be reviewed on a first-come, first-served basis which also includes study amendment applications and continuing renewals. Therefore, the ORC highly recommends accurate and timely submission of the application and attachments to account for this review process.
- Investigators may receive requests from the ORC to make changes to their submission during the pre-review screening process and/or after formal IRB Member/Board review. These comments must be addressed prior to IRB approval and a deadline for response will be provided. Once satisfactory responses are received, the ORC will move the application to the next step in the review process.

Complete Education & Training
- Required Human Subjects Research training - All investigators and staff engaged in human subjects research are required to complete human subjects research training.
- UCR personnel should access the Collaborative Institutional Training Initiative (CITI) online courses. UCR Researchers should select the courses that specifically pertain to their field or involvement in the human subjects research project. Options include:
- Social & behavioral research investigators
- Biomedical Research Investigators
- Research with data or laboratory specimens ONLY
- Community Members/Partners (unaffiliated with UCR or other institutions) should complete the CIRTification Online course.
- UCR personnel should access the Collaborative Institutional Training Initiative (CITI) online courses. UCR Researchers should select the courses that specifically pertain to their field or involvement in the human subjects research project. Options include:
- Completed human subjects research training is valid for 3 years and must be renewed as long as the researcher is engaged in human subjects research.
- IRB approval cannot be issued until all listed research personnel on the IRB protocol roster have completed the required training.
- The Lead Researcher is responsible for ensuring the completion of Human Subjects Research Training for all research personnel (including staff, students, community members/partners, etc.) engaged in research activities.
Additional Information
Researchers and committee members can find more information in the IRB section of the Resources page.
IRB Consults & Contact Information
University of California, Riverside
Office of Research Integrity
Phone: 951-827-4802
IRB@ucr.edu
ORI offers IRB consultations for UCR investigators and research staff who are preparing IRB submissions (new studies, amendments) or preparing responses to review comments. Please email us at IRB@ucr.edu to set up an appointment to speak with an ORI representative.